当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Perturbation in Long-Range Contacts Modulates the Kinetics of Amyloid Formation in α-Synuclein Familial Mutants
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acschemneuro.7b00149 Priyatosh Ranjan 1 , Ashutosh Kumar 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acschemneuro.7b00149 Priyatosh Ranjan 1 , Ashutosh Kumar 1
Affiliation
|
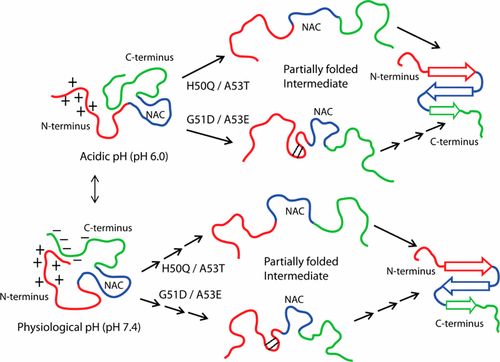
The characteristic cross-β-sheet-rich amyloid fibril formation by intrinsically disordered α-synuclein proteins is one of the pathological hallmarks of Parkinson’s disease. Although unstructured in solution, the presence of autoinhibitory long-range contacts in monomeric form prevents protein aggregation. Out of the various factors that affect the rate of amyloid formation, familial mutations play an important role in α-synuclein aggregation. Even though these mutations are believed to form an aggregation-prone intermediate by perturbing these contacts, the correlation between perturbation and rate of fibril formation is not very straightforward. A combination of solution and solid-state NMR in conjunction with other biophysical methods has been used to identify the underlying mechanism behind the anomaly in the rate of aggregation for the novel mutants H50Q (fast aggregating) and G51D (slow aggregating). Perturbation of long-range contacts at the mutation sites and C-termini in all of the six familial mutants of α-synuclein during the diseased condition (acidic pH) was observed. These contacts get rearranged at physiological pH resulting in the shielding of mutation sites. Additional contacts at the mutation site in a slow aggregating mutant could be the reason for slower aggregation. Indeed, these contacts provide more rigidity to the monomeric G51D. Nonetheless, these mutations did not alter the overall secondary structure. The differential pattern of the long-range contacts at the monomeric level resulted in the perturbation of the fibrillar-core region, which was evident in the solid-state NMR spectra. Our results provide valuable insights in understanding the effect of long-range contacts on the aggregation of α-synuclein and its mutants.
中文翻译:

远程接触中的扰动调节α-突触核蛋白家族突变体中淀粉样蛋白形成的动力学。
内在无序的α-突触核蛋白蛋白形成的富含β-折叠片的淀粉样原纤维具有特征性,是帕金森氏病的病理标志之一。尽管在溶液中是非结构化的,但单体形式的自抑制性远距离接触的存在可防止蛋白质聚集。在影响淀粉样蛋白形成速率的各种因素中,家族突变在α-突触核蛋白聚集中起重要作用。尽管据信这些突变会通过扰动这些接触而形成易于聚集的中间体,但扰动与原纤维形成速率之间的相关性并不是很直接。溶液和固态NMR以及其他生物物理方法的结合已用于确定新型突变体H50Q(快速聚集)和G51D(缓慢聚集)的聚集速率异常背后的潜在机制。在患病状态(酸性pH)期间,在α-突触核蛋白的所有六个家族突变体中,均观察到突变位点和C-末端的长期接触受到干扰。这些接触在生理pH下重新排列,从而屏蔽了突变位点。缓慢聚集的突变体中突变位点的其他接触可能是缓慢聚集的原因。实际上,这些触点为单体G51D提供了更高的刚性。尽管如此,这些突变并未改变整体二级结构。在单体水平上的远距离接触的差异模式导致了纤芯核心区域的扰动,这在固态NMR光谱中很明显。我们的结果为理解远程接触对α-突触核蛋白及其突变体聚集的影响提供了宝贵的见识。
更新日期:2017-08-14
中文翻译:

远程接触中的扰动调节α-突触核蛋白家族突变体中淀粉样蛋白形成的动力学。
内在无序的α-突触核蛋白蛋白形成的富含β-折叠片的淀粉样原纤维具有特征性,是帕金森氏病的病理标志之一。尽管在溶液中是非结构化的,但单体形式的自抑制性远距离接触的存在可防止蛋白质聚集。在影响淀粉样蛋白形成速率的各种因素中,家族突变在α-突触核蛋白聚集中起重要作用。尽管据信这些突变会通过扰动这些接触而形成易于聚集的中间体,但扰动与原纤维形成速率之间的相关性并不是很直接。溶液和固态NMR以及其他生物物理方法的结合已用于确定新型突变体H50Q(快速聚集)和G51D(缓慢聚集)的聚集速率异常背后的潜在机制。在患病状态(酸性pH)期间,在α-突触核蛋白的所有六个家族突变体中,均观察到突变位点和C-末端的长期接触受到干扰。这些接触在生理pH下重新排列,从而屏蔽了突变位点。缓慢聚集的突变体中突变位点的其他接触可能是缓慢聚集的原因。实际上,这些触点为单体G51D提供了更高的刚性。尽管如此,这些突变并未改变整体二级结构。在单体水平上的远距离接触的差异模式导致了纤芯核心区域的扰动,这在固态NMR光谱中很明显。我们的结果为理解远程接触对α-突触核蛋白及其突变体聚集的影响提供了宝贵的见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号