Nature Chemistry ( IF 19.2 ) Pub Date : 2017-08-14 , DOI: 10.1038/nchem.2842 Michael P. Burke , Stephen J. Klippenstein
|
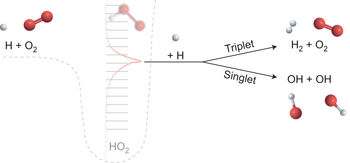
Termolecular association reactions involve ephemeral collision complexes—formed from the collision of two molecules—that collide with a third and chemically inert ‘bath gas’ molecule that simply transfers energy to/from the complex. These collision complexes are generally not thought to react chemically on collision with a third molecule in the gas-phase systems of combustion and planetary atmospheres. Such ‘chemically termolecular’ reactions, in which all three molecules are involved in bond making and/or breaking, were hypothesized long ago in studies establishing radical chain branching mechanisms, but were later concluded to be unimportant. Here, with data from ab initio master equation and kinetic-transport simulations, we reveal that reactions of H + O2 collision complexes with other radicals constitute major kinetic pathways under common combustion situations. These reactions are also found to influence flame propagation speeds, a common measure of global reactivity. Analogous chemically termolecular reactions mediated by ephemeral collision complexes are probably of significance in various combustion and planetary environments.
中文翻译:

临时碰撞配合物介导影响系统化学的化学分子转化
分子间缔合反应涉及短暂的碰撞复合物(由两个分子的碰撞形成),它们与第三个化学惰性的“浴气体”分子发生碰撞,该分子简单地将能量转移至复合物或从复合物中转移能量。通常认为这些碰撞复合物在燃烧和行星大气的气相系统中与第三分子碰撞时不会发生化学反应。早在建立自由基链支化机理的研究中就假设了这样的“化学分子”反应,其中所有三个分子均参与键的形成和/或断裂,但后来得出的结论并不重要。在这里,利用从头算主方程和动力学传输模拟得到的数据,我们揭示了H + O 2的反应在常见的燃烧情况下,与其他自由基的碰撞配合物构成了主要的动力学路径。还发现这些反应会影响火焰传播速度,这是整体反应性的一种常用度量。短暂碰撞配合物介导的类似化学分子反应在各种燃烧和行星环境中可能具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号