Nature Chemistry ( IF 19.2 ) Pub Date : 2017-08-14 , DOI: 10.1038/nchem.2835 Christopher K Hill 1 , John F Hartwig 1
|
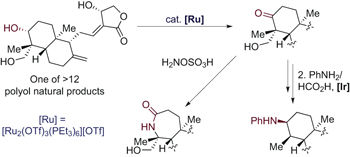
Polyoxygenated hydrocarbons that bear one or more hydroxyl groups comprise a large set of natural and synthetic compounds, often with potent biological activity. In synthetic chemistry, alcohols are important precursors to carbonyl groups, which then can be converted into a wide range of oxygen- or nitrogen-based functionality. Therefore, the selective conversion of a single hydroxyl group in natural products into a ketone would enable the selective introduction of unnatural functionality. However, the methods known to convert a simple alcohol, or even an alcohol in a molecule that contains multiple protected functional groups, are not suitable for selective reactions of complex polyol structures. We present a new ruthenium catalyst with a unique efficacy for the selective oxidation of a single hydroxyl group among many in unprotected polyol natural products. This oxidation enables the introduction of nitrogen-based functional groups into such structures that lack nitrogen atoms and enables a selective alcohol epimerization by stepwise or reversible oxidation and reduction.
中文翻译:

通过转移氢化实现复杂多元醇的位点选择性氧化、胺化和差向异构化反应
带有一个或多个羟基的多氧化碳氢化合物包含一大组天然和合成化合物,通常具有有效的生物活性。在合成化学中,醇是羰基的重要前体,然后可以将其转化为各种氧基或氮基官能团。因此,选择性地将天然产物中的单个羟基转化为酮将能够选择性地引入非天然官能团。然而,已知的转化简单醇或什至分子中含有多个受保护官能团的醇的方法并不适合复杂多元醇结构的选择性反应。我们提出了一种新型钌催化剂,对于未受保护的多元醇天然产物中的许多单个羟基的选择性氧化具有独特的功效。这种氧化能够将氮基官能团引入到缺乏氮原子的结构中,并能够通过逐步或可逆的氧化和还原进行选择性醇差向异构化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号