Journal of Catalysis ( IF 7.3 ) Pub Date : 2017-08-12 , DOI: 10.1016/j.jcat.2017.07.018 Fatima Jalid , Tuhin Suvra Khan , Fasil Qayoom Mir , M. Ali Haider
|
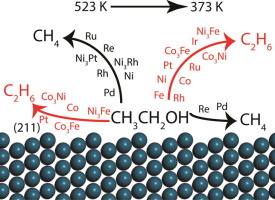
Platform molecules obtained from biomass are often found enriched with oxygen and require a suitable deoxygenation process for their conversion into high value chemicals and fuels. In this study, a combined density functional theory (DFT) and microkinetic model (MKM) based approach was utilized to understand the catalytic activity and selectivity trends observed for the hydrodeoxygenation (HDO) reactions of biomass-derived platform molecules on supported metal catalysts. This understanding was developed on modeling the CO cleavage reaction of ethanol molecule over the stepped (2 1 1) surface of the metal catalysts using both carbon and oxygen binding energies as the descriptors. The two descriptor MKM showed that the activity for ethanol conversion to ethane at 523 K for a range of metal catalysts follows the order; Co > Ru > Ir > Rh > Ni > Fe > Pt > Pd > Cu > Re > Ag > Au. In general, as observed in the experiments of previous reports, the C
C cleavage in ethanol to produce methane shows higher turnover frequencies (TOFs) with negligible ethane formation. In contrast, since the focus of the MKM developed here was on the stepped surfaces, significant conversion towards the formation of ethane was calculated. The selectivity towards the HDO product was further improved on reducing the reaction temperature to 373 K. A range of metal catalysts viz. Cu, Pt, Rh, Ir, Ru, Ni and Co was observed to yield ethane selectively at 373 K, albeit with significantly reduced conversion. While the shift in selectivity towards the HDO product was at the expense of catalyst reactivity, general conclusions on the design of a selective metal HDO catalyst were drawn, further extending it to the design of a bimetallic alloy. In the screening of metal alloys, three specific catalysts (Co3Ni, Co3Fe and Ni3Fe) were observed to show the maximum turnover of ethane (10−3 s−1) with high selectivity. More specifically, the MKM developed for both metal and bimetallic catalysts provided insights into the reactivity trends observed in recent experimental HDO studies on biomass-derived model phenolic compounds.
中文翻译:

通过阶梯表面上的乙醇反应了解金属和双金属合金催化剂的加氢脱氧反应性趋势
通常发现从生物质获得的平台分子富含氧气,并且需要适当的脱氧过程才能将其转化为高价值的化学品和燃料。在这项研究中,结合了基于密度泛函理论(DFT)和微动力学模型(MKM)的方法,以了解在负载金属催化剂上生物质衍生的平台分子加氢脱氧(HDO)反应的催化活性和选择性趋势。这种理解是在对C语言进行建模时获得的使用碳和氧的结合能作为描述符,在金属催化剂的阶梯状(2 1 1)表面上进行乙醇分子的O裂解反应。两个描述符MKM表明,对于一系列金属催化剂,在523 K下乙醇转化为乙烷的活性遵循以下顺序:Co> Ru> Ir> Rh> Ni> Fe> Pt> Pd> Cu> Re> Ag> Au 通常,如先前报告的实验中所观察到的,C
乙醇中的C裂解产生甲烷显示出更高的周转频率(TOF),而乙烷的形成可忽略不计。相比之下,由于此处开发的MKM的焦点位于台阶表面上,因此计算出向乙烷形成的明显转化。通过将反应温度降低至373 K,进一步提高了对HDO产物的选择性。观察到Cu,Pt,Rh,Ir,Ru,Ni和Co在373 K选择性地产生乙烷,尽管转化率明显降低。尽管向HDO产物的选择性转移是以牺牲催化剂的反应性为代价的,但还是得出了关于选择性金属HDO催化剂设计的一般结论,并将其进一步扩展到双金属合金的设计中。在金属合金的筛选中,三种特定的催化剂(钴观察到3 Ni,Co 3 Fe和Ni 3 Fe )以高选择性显示乙烷(10 -3 s -1)的最大周转率。更具体地说,为金属和双金属催化剂开发的MKM提供了对最近对生物质衍生的模型酚类化合物进行的HDO实验研究中观察到的反应性趋势的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号