当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrophilic and Cell-Penetrable Pyrrolidinyl Peptide Nucleic Acid via Post-synthetic Modification with Hydrophilic Side Chains
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00308 Haruthai Pansuwan 1 , Boonsong Ditmangklo , Chotima Vilaivan , Banphot Jiangchareon , Porntip Pan-In , Supason Wanichwecharungruang , Tanapat Palaga , Thanesuan Nuanyai 2 , Chaturong Suparpprom 1 , Tirayut Vilaivan
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00308 Haruthai Pansuwan 1 , Boonsong Ditmangklo , Chotima Vilaivan , Banphot Jiangchareon , Porntip Pan-In , Supason Wanichwecharungruang , Tanapat Palaga , Thanesuan Nuanyai 2 , Chaturong Suparpprom 1 , Tirayut Vilaivan
Affiliation
|
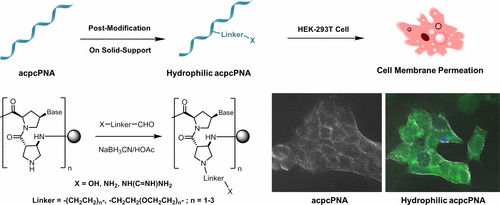
Peptide nucleic acid (PNA) is a nucleic acid mimic in which the deoxyribose–phosphate was replaced by a peptide-like backbone. The absence of negative charge in the PNA backbone leads to several unique behaviors including a stronger binding and salt independency of the PNA–DNA duplex stability. However, PNA possesses poor aqueous solubility and cannot directly penetrate cell membranes. These are major obstacles that limit in vivo applications of PNA. In previous strategies, the PNA can be conjugated to macromolecular carriers or modified with positively charged side chains such as guanidinium groups to improve the aqueous solubility and cell permeability. In general, a preformed modified PNA monomer was required. In this study, a new approach for post-synthetic modification of PNA backbone with one or more hydrophilic groups was proposed. The PNA used in this study was the conformationally constrained pyrrolidinyl PNA with prolyl-2-aminocyclopentanecarboxylic acid dipeptide backbone (acpcPNA) that shows several advantages over the conventional PNA. The aldehyde modifiers carrying different linkers (alkylene and oligo(ethylene glycol)) and end groups (−OH, −NH2, and guanidinium) were synthesized and attached to the backbone of modified acpcPNA by reductive alkylation. The hybrids between the modified acpcPNAs and DNA exhibited comparable or superior thermal stability with base-pairing specificity similar to those of unmodified acpcPNA. Moreover, the modified apcPNAs also showed the improvement of aqueous solubility (10–20 folds compared to unmodified PNA) and readily penetrate cell membranes without requiring any special delivery agents. This study not only demonstrates the practicality of the proposed post-synthetic modification approach for PNA modification, which could be readily applied to other systems, but also opens up opportunities for using pyrrolidinyl PNA in various applications such as intracellular RNA sensing, specific gene detection, and antisense and antigene therapy.
中文翻译:

通过亲水侧链的后合成修饰的亲水性和可穿透细胞的吡咯烷基肽核酸。
肽核酸(PNA)是一种核酸模拟物,其中的脱氧核糖磷酸被一个类似肽的骨架所取代。PNA主链上没有负电荷会导致几种独特的行为,包括更强的结合力和PNA-DNA双链体稳定性的盐独立性。但是,PNA的水溶性差,不能直接穿透细胞膜。这些是限制PNA在体内应用的主要障碍。在以前的策略中,PNA可以与大分子载体缀合或用带正电荷的侧链(例如胍基)修饰,以改善水溶性和细胞渗透性。通常,需要预制的改性PNA单体。在这项研究中,提出了一种利用一个或多个亲水基团对PNA主链进行后合成修饰的新方法。这项研究中使用的PNA是具有脯氨酰-2-氨基环戊烷羧酸二肽主链(acpcPNA)的构象受限的吡咯烷基PNA,与常规PNA相比,显示出一些优势。带有不同接头(亚烷基和低聚乙二醇)和端基(-OH,-NH)的醛改性剂2个,和胍基)被合成并通过还原烷基化连接到修饰的acpcPNA的主链上。修饰的acpccPNA和DNA之间的杂种表现出相当的或更高的热稳定性,其碱基配对特异性与未修饰的acpcPNA相似。此外,修饰的apcPNA还显示出水溶性的改善(与未修饰的PNA相比,提高了10-20倍),并且不需要任何特殊的传递剂即可轻松穿透细胞膜。这项研究不仅证明了拟议的PNA修饰后合成修饰方法的实用性,可以很容易地应用于其他系统,而且为在各种应用中使用吡咯烷基PNA提供了机会,例如细胞内RNA传感,特异性基因检测,以及反义和抗原疗法。
更新日期:2017-08-12
中文翻译:

通过亲水侧链的后合成修饰的亲水性和可穿透细胞的吡咯烷基肽核酸。
肽核酸(PNA)是一种核酸模拟物,其中的脱氧核糖磷酸被一个类似肽的骨架所取代。PNA主链上没有负电荷会导致几种独特的行为,包括更强的结合力和PNA-DNA双链体稳定性的盐独立性。但是,PNA的水溶性差,不能直接穿透细胞膜。这些是限制PNA在体内应用的主要障碍。在以前的策略中,PNA可以与大分子载体缀合或用带正电荷的侧链(例如胍基)修饰,以改善水溶性和细胞渗透性。通常,需要预制的改性PNA单体。在这项研究中,提出了一种利用一个或多个亲水基团对PNA主链进行后合成修饰的新方法。这项研究中使用的PNA是具有脯氨酰-2-氨基环戊烷羧酸二肽主链(acpcPNA)的构象受限的吡咯烷基PNA,与常规PNA相比,显示出一些优势。带有不同接头(亚烷基和低聚乙二醇)和端基(-OH,-NH)的醛改性剂2个,和胍基)被合成并通过还原烷基化连接到修饰的acpcPNA的主链上。修饰的acpccPNA和DNA之间的杂种表现出相当的或更高的热稳定性,其碱基配对特异性与未修饰的acpcPNA相似。此外,修饰的apcPNA还显示出水溶性的改善(与未修饰的PNA相比,提高了10-20倍),并且不需要任何特殊的传递剂即可轻松穿透细胞膜。这项研究不仅证明了拟议的PNA修饰后合成修饰方法的实用性,可以很容易地应用于其他系统,而且为在各种应用中使用吡咯烷基PNA提供了机会,例如细胞内RNA传感,特异性基因检测,以及反义和抗原疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号