Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nivolumab versus standard, single-agent therapy of investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial.
The Lancet ( IF 98.4 ) Pub Date : 2017-08-01 , DOI: 10.1016/s1470-2045(17)30421-7 Kevin J Harrington , Robert L Ferris , George Blumenschein , A Dimitrios Colevas , Jérôme Fayette , Lisa Licitra , Stefan Kasper , Caroline Even , Everett E Vokes , Francis Worden , Nabil F Saba , Naomi Kiyota , Robert Haddad , Makoto Tahara , Viktor Grünwald , James W Shaw , Manish Monga , Mark Lynch , Fiona Taylor , Michael DeRosa , Laura Morrissey , Kim Cocks , Maura L Gillison , Joël Guigay
The Lancet ( IF 98.4 ) Pub Date : 2017-08-01 , DOI: 10.1016/s1470-2045(17)30421-7 Kevin J Harrington , Robert L Ferris , George Blumenschein , A Dimitrios Colevas , Jérôme Fayette , Lisa Licitra , Stefan Kasper , Caroline Even , Everett E Vokes , Francis Worden , Nabil F Saba , Naomi Kiyota , Robert Haddad , Makoto Tahara , Viktor Grünwald , James W Shaw , Manish Monga , Mark Lynch , Fiona Taylor , Michael DeRosa , Laura Morrissey , Kim Cocks , Maura L Gillison , Joël Guigay
|
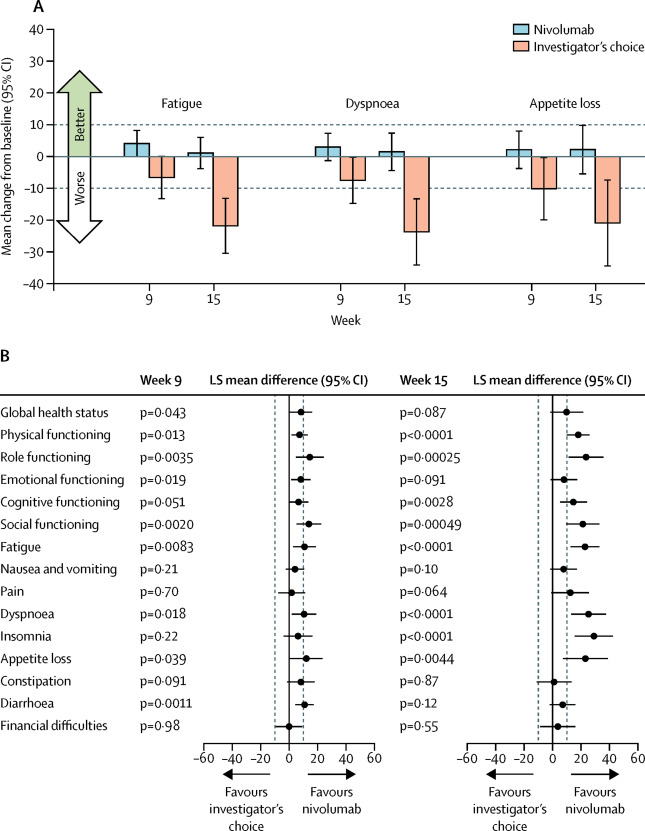
Patients with platinum-refractory recurrent or metastatic squamous cell carcinoma of the head and neck have few treatment options and poor prognosis. Nivolumab significantly improved survival of this patient population when compared with standard single-agent therapy of investigator's choice in Checkmate 141; here we report the effect of nivolumab on patient-reported outcomes (PROs).
中文翻译:

Nivolumab与标准的单药治疗相比,研究者在复发性或转移性头颈部鳞状细胞癌中的选择(CheckMate 141):与健康相关的生活质量来自一项随机的3期临床试验。
患有铂难治性复发性或转移性头颈部鳞状细胞癌的患者治疗选择少且预后差。与研究人员在Checkmate 141中选择的标准单药疗法相比,Nivolumab显着改善了该患者人群的生存率;在这里,我们报告了nivolumab对患者报告的结局(PROs)的影响。
更新日期:2017-08-10
中文翻译:

Nivolumab与标准的单药治疗相比,研究者在复发性或转移性头颈部鳞状细胞癌中的选择(CheckMate 141):与健康相关的生活质量来自一项随机的3期临床试验。
患有铂难治性复发性或转移性头颈部鳞状细胞癌的患者治疗选择少且预后差。与研究人员在Checkmate 141中选择的标准单药疗法相比,Nivolumab显着改善了该患者人群的生存率;在这里,我们报告了nivolumab对患者报告的结局(PROs)的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号