Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study.
The Lancet ( IF 98.4 ) Pub Date : 2017-08-01 , DOI: 10.1016/s1470-2045(17)30416-3 Alexander E Perl , Jessica K Altman , Jorge Cortes , Catherine Smith , Mark Litzow , Maria R Baer , David Claxton , Harry P Erba , Stan Gill , Stuart Goldberg , Joseph G Jurcic , Richard A Larson , Chaofeng Liu , Ellen Ritchie , Gary Schiller , Alexander I Spira , Stephen A Strickland , Raoul Tibes , Celalettin Ustun , Eunice S Wang , Robert Stuart , Christoph Röllig , Andreas Neubauer , Giovanni Martinelli , Erkut Bahceci , Mark Levis
The Lancet ( IF 98.4 ) Pub Date : 2017-08-01 , DOI: 10.1016/s1470-2045(17)30416-3 Alexander E Perl , Jessica K Altman , Jorge Cortes , Catherine Smith , Mark Litzow , Maria R Baer , David Claxton , Harry P Erba , Stan Gill , Stuart Goldberg , Joseph G Jurcic , Richard A Larson , Chaofeng Liu , Ellen Ritchie , Gary Schiller , Alexander I Spira , Stephen A Strickland , Raoul Tibes , Celalettin Ustun , Eunice S Wang , Robert Stuart , Christoph Röllig , Andreas Neubauer , Giovanni Martinelli , Erkut Bahceci , Mark Levis
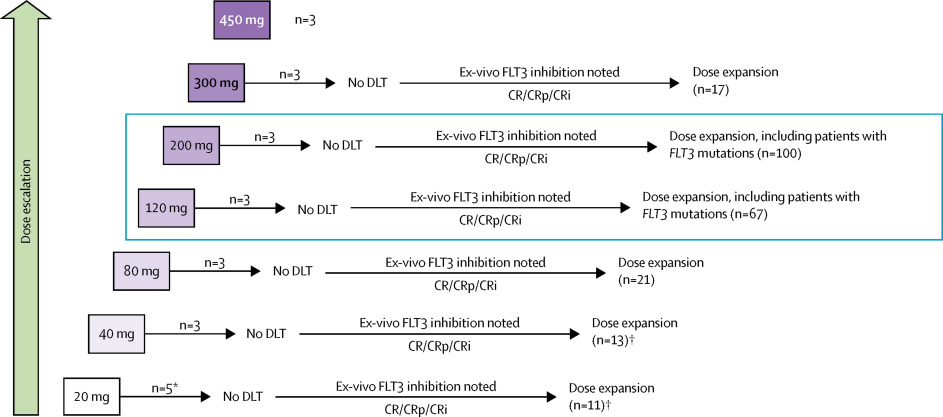
|
Internal tandem duplication mutations in FLT3 are common in acute myeloid leukaemia and are associated with rapid relapse and short overall survival. The clinical benefit of FLT3 inhibitors in patients with acute myeloid leukaemia has been limited by rapid generation of resistance mutations, particularly in codon Asp835 (D835). We aimed to assess the highly selective oral FLT3 inhibitor gilteritinib in patients with relapsed or refractory acute myeloid leukaemia.
中文翻译:

吉尔替尼对复发性或难治性急性髓性白血病的选择性抑制FLT3:一项多中心,首次在人体内,开放标签的1-2期研究。
FLT3的内部串联重复突变在急性粒细胞白血病中很常见,并且与复发快和总生存期短有关。FLT3抑制剂在急性髓性白血病患者中的临床获益已受到耐药性突变(尤其是密码子Asp835(D835))快速生成的限制。我们旨在评估复发性或难治性急性髓性白血病患者中的高选择性口服FLT3抑制剂gilteritinib。
更新日期:2017-08-10
中文翻译:

吉尔替尼对复发性或难治性急性髓性白血病的选择性抑制FLT3:一项多中心,首次在人体内,开放标签的1-2期研究。
FLT3的内部串联重复突变在急性粒细胞白血病中很常见,并且与复发快和总生存期短有关。FLT3抑制剂在急性髓性白血病患者中的临床获益已受到耐药性突变(尤其是密码子Asp835(D835))快速生成的限制。我们旨在评估复发性或难治性急性髓性白血病患者中的高选择性口服FLT3抑制剂gilteritinib。











































 京公网安备 11010802027423号
京公网安备 11010802027423号