当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering Intracellularly Retained Gaussia Luciferase Reporters for Improved Biosensing and Molecular Imaging Applications
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-08-10 00:00:00 , DOI: 10.1021/acschembio.7b00454 Shuchi Gaur 1 , Aarohi Bhargava-Shah 1 , Sharon Hori 1 , Rayhaneh Afjei 1 , Thillai V. Sekar 1 , Sanjiv S. Gambhir 1 , Tarik F. Massoud 1 , Ramasamy Paulmurugan 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-08-10 00:00:00 , DOI: 10.1021/acschembio.7b00454 Shuchi Gaur 1 , Aarohi Bhargava-Shah 1 , Sharon Hori 1 , Rayhaneh Afjei 1 , Thillai V. Sekar 1 , Sanjiv S. Gambhir 1 , Tarik F. Massoud 1 , Ramasamy Paulmurugan 1
Affiliation
|
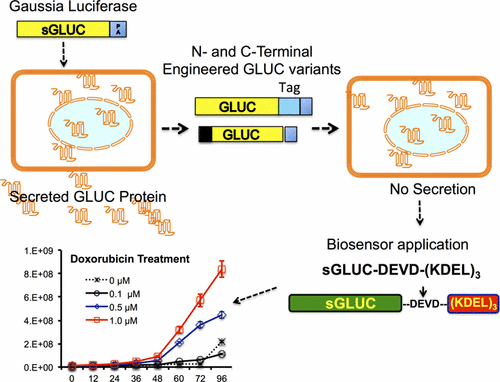
Gaussia luciferase (GLUC) is a bioluminescent reporter protein of increasing importance. As a secretory protein, it has increased sensitivity in vitro and in vivo (∼20 000-fold, and ∼1000-fold, respectively) over its competitor, secreted alkaline phosphatase. Unfortunately, this same advantageous secretory nature of GLUC limits its usefulness for many other possible intracellular applications, e.g., imaging signaling pathways in intact cells, in vivo imaging, and in developing molecular imaging biosensors to study protein–protein interactions and protein folding. Hence, to widen the research applications of GLUC, we developed engineered variants that increase its intracellular retention both by modifying the N-terminal secretory signal peptide and by tagging additional sequences to its C-terminal region. We found that when GLUC was expressed in mammalian cells, its N-terminal secretory signal peptide comprising amino acids 1–16 was essential for GLUC folding and functional activity in addition to its inherent secretory property. Modification of the C-terminus of GLUC by tagging a four amino acid (KDEL) endoplasmic reticulum targeting peptide in multiple repeats significantly improved its intracellular retention, with little impact on its folding and enzymatic activity. We used stable cells expressing this engineered GLUC with KDEL repeats to monitor chemically induced endoplasmic reticulum stress on cells. Additionally, we engineered an apoptotic sensor using modified variants of GLUC containing a four amino acid caspase substrate peptide (DEVD) between the GLUC protein and the KDEL repeats. Its use in cell culture resulted in increased GLUC secretion in the growth medium when cells were treated with the chemotherapeutic drugs doxorubicin, paclitaxel, and carboplatin. We thus successfully engineered a new variant GLUC protein that is retained inside cells rather than secreted extracellularly. We validated this novel reporter by incorporating it in biosensors for detection of cellular endoplasmic reticulum stress and caspase activation. This new molecularly engineered enzymatic reporter has the potential for widespread applications in biological research.
中文翻译:

工程细胞内保留的高斯荧光素酶报道基因,以改善生物传感和分子成像应用
高斯荧光素酶(GLUC)是一种越来越重要的生物发光报告蛋白。作为一种分泌蛋白,它比其竞争对手分泌的碱性磷酸酶具有更高的体外和体内敏感性(分别为约20000倍和约1000倍)。不幸的是,GLUC的这种同样有利的分泌特性限制了它在许多其他可能的细胞内应用中的有用性,例如在体内完整细胞中的成像信号通路成像,以及开发分子成像生物传感器来研究蛋白质间相互作用和蛋白质折叠。因此,为了拓宽GLUC的研究应用范围,我们开发了经过工程改造的变体,既可以通过修饰N末端分泌信号肽,也可以通过将其他序列标记到其C末端区域来增加其细胞内保留。我们发现,当GLUC在哺乳动物细胞中表达时,它的N末端分泌信号肽(包含氨基酸1-16)除了具有固有的分泌特性外,对于GLUC折叠和功能活性也是必不可少的。通过在多个重复序列中标记四个氨基酸(KDEL)内质网靶向肽修饰GLUC的C端,可显着改善其细胞内滞留性,对其折叠和酶活性几乎没有影响。我们使用表达带有KDEL重复序列的工程化GLUC的稳定细胞来监测化学诱导的内质网对细胞的应激。此外,我们使用修饰的GLUC变异体设计了一种凋亡传感器,该变异体在GLUC蛋白和KDEL重复序列之间包含四个氨基酸的半胱天冬酶底物肽(DEVD)。当用化学治疗药物阿霉素,紫杉醇和卡铂处理细胞时,其在细胞培养中的使用导致生长培养基中GLUC分泌增加。因此,我们成功地设计了一种新的变体GLUC蛋白,该蛋白保留在细胞内而不是分泌到细胞外。我们通过将其纳入生物传感器来检测细胞内质网应激和caspase激活,从而验证了该新型报道基因。
更新日期:2017-08-10
中文翻译:

工程细胞内保留的高斯荧光素酶报道基因,以改善生物传感和分子成像应用
高斯荧光素酶(GLUC)是一种越来越重要的生物发光报告蛋白。作为一种分泌蛋白,它比其竞争对手分泌的碱性磷酸酶具有更高的体外和体内敏感性(分别为约20000倍和约1000倍)。不幸的是,GLUC的这种同样有利的分泌特性限制了它在许多其他可能的细胞内应用中的有用性,例如在体内完整细胞中的成像信号通路成像,以及开发分子成像生物传感器来研究蛋白质间相互作用和蛋白质折叠。因此,为了拓宽GLUC的研究应用范围,我们开发了经过工程改造的变体,既可以通过修饰N末端分泌信号肽,也可以通过将其他序列标记到其C末端区域来增加其细胞内保留。我们发现,当GLUC在哺乳动物细胞中表达时,它的N末端分泌信号肽(包含氨基酸1-16)除了具有固有的分泌特性外,对于GLUC折叠和功能活性也是必不可少的。通过在多个重复序列中标记四个氨基酸(KDEL)内质网靶向肽修饰GLUC的C端,可显着改善其细胞内滞留性,对其折叠和酶活性几乎没有影响。我们使用表达带有KDEL重复序列的工程化GLUC的稳定细胞来监测化学诱导的内质网对细胞的应激。此外,我们使用修饰的GLUC变异体设计了一种凋亡传感器,该变异体在GLUC蛋白和KDEL重复序列之间包含四个氨基酸的半胱天冬酶底物肽(DEVD)。当用化学治疗药物阿霉素,紫杉醇和卡铂处理细胞时,其在细胞培养中的使用导致生长培养基中GLUC分泌增加。因此,我们成功地设计了一种新的变体GLUC蛋白,该蛋白保留在细胞内而不是分泌到细胞外。我们通过将其纳入生物传感器来检测细胞内质网应激和caspase激活,从而验证了该新型报道基因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号