Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade.
Cell ( IF 45.5 ) Pub Date : 2017-Sep-07 , DOI: 10.1016/j.cell.2017.07.024 Spencer C. Wei , Jacob H. Levine , Alexandria P. Cogdill , Yang Zhao , Nana-Ama A.S. Anang , Miles C. Andrews , Padmanee Sharma , Jing Wang , Jennifer A. Wargo , Dana Pe’er , James P. Allison
Cell ( IF 45.5 ) Pub Date : 2017-Sep-07 , DOI: 10.1016/j.cell.2017.07.024 Spencer C. Wei , Jacob H. Levine , Alexandria P. Cogdill , Yang Zhao , Nana-Ama A.S. Anang , Miles C. Andrews , Padmanee Sharma , Jing Wang , Jennifer A. Wargo , Dana Pe’er , James P. Allison
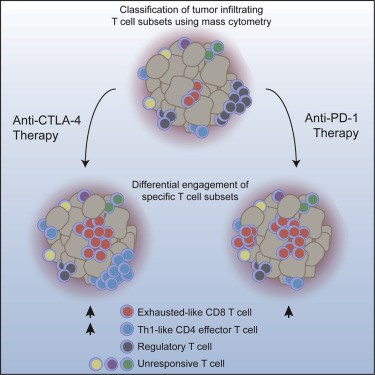
|
Immune-checkpoint blockade is able to achieve durable responses in a subset of patients; however, we lack a satisfying comprehension of the underlying mechanisms of anti-CTLA-4- and anti-PD-1-induced tumor rejection. To address these issues, we utilized mass cytometry to comprehensively profile the effects of checkpoint blockade on tumor immune infiltrates in human melanoma and murine tumor models. These analyses reveal a spectrum of tumor-infiltrating T cell populations that are highly similar between tumor models and indicate that checkpoint blockade targets only specific subsets of tumor-infiltrating T cell populations. Anti-PD-1 predominantly induces the expansion of specific tumor-infiltrating exhausted-like CD8 T cell subsets. In contrast, anti-CTLA-4 induces the expansion of an ICOS+ Th1-like CD4 effector population in addition to engaging specific subsets of exhausted-like CD8 T cells. Thus, our findings indicate that anti-CTLA-4 and anti-PD-1 checkpoint-blockade-induced immune responses are driven by distinct cellular mechanisms.
中文翻译:

不同的细胞机制是抗CTLA-4和抗PD-1检查点封锁的基础。
免疫检查点封锁能够在部分患者中实现持久的反应。但是,我们对抗CTLA-4和抗PD-1诱导的肿瘤排斥的潜在机制缺乏令人满意的理解。为了解决这些问题,我们利用细胞计数技术全面分析了检查点封锁对人黑素瘤和鼠类肿瘤模型中肿瘤免疫浸润的影响。这些分析揭示了在肿瘤模型之间高度相似的肿瘤浸润性T细胞群体,并表明检查点封锁仅针对肿瘤浸润性T细胞群体的特定子集。抗PD-1主要诱导特定的肿瘤浸润精疲力竭CD8 T细胞亚群的扩增。相反,抗CTLA-4可诱导ICOS +的扩增Th1类CD4效应子种群,除了参与了疲惫的CD8 T细胞的特定子集。因此,我们的研究结果表明抗CTLA-4和抗PD-1检查点封锁诱导的免疫反应是由不同的细胞机制驱动的。
更新日期:2017-08-10
中文翻译:

不同的细胞机制是抗CTLA-4和抗PD-1检查点封锁的基础。
免疫检查点封锁能够在部分患者中实现持久的反应。但是,我们对抗CTLA-4和抗PD-1诱导的肿瘤排斥的潜在机制缺乏令人满意的理解。为了解决这些问题,我们利用细胞计数技术全面分析了检查点封锁对人黑素瘤和鼠类肿瘤模型中肿瘤免疫浸润的影响。这些分析揭示了在肿瘤模型之间高度相似的肿瘤浸润性T细胞群体,并表明检查点封锁仅针对肿瘤浸润性T细胞群体的特定子集。抗PD-1主要诱导特定的肿瘤浸润精疲力竭CD8 T细胞亚群的扩增。相反,抗CTLA-4可诱导ICOS +的扩增Th1类CD4效应子种群,除了参与了疲惫的CD8 T细胞的特定子集。因此,我们的研究结果表明抗CTLA-4和抗PD-1检查点封锁诱导的免疫反应是由不同的细胞机制驱动的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号