JAMA Psychiatry ( IF 25.8 ) Pub Date : 2017-08-01 , DOI: 10.1001/jamapsychiatry.2017.1574 Richard S. E. Keefe 1 , Vicki G. Davis 2 , Philip D. Harvey 3 , Alexandra S. Atkins 2 , George M. Haig 4 , Owen Hagino 5 , Stephen Marder 6 , Dana C. Hilt 7 , Daniel Umbricht 8
|
Importance Patients’ previous experience with performance-based cognitive tests in clinical trials for cognitive impairment associated with schizophrenia can create practice-related improvements. Placebo-controlled trials for cognitive impairment associated with schizophrenia are at risk for these practice effects, which can be difficult to distinguish from placebo effects.
Objectives To conduct a systematic evaluation of the magnitude of practice effects on the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB) in cognitive impairment associated with schizophrenia and to examine which demographic, clinical, and cognitive characteristics were associated with improvement in placebo conditions.
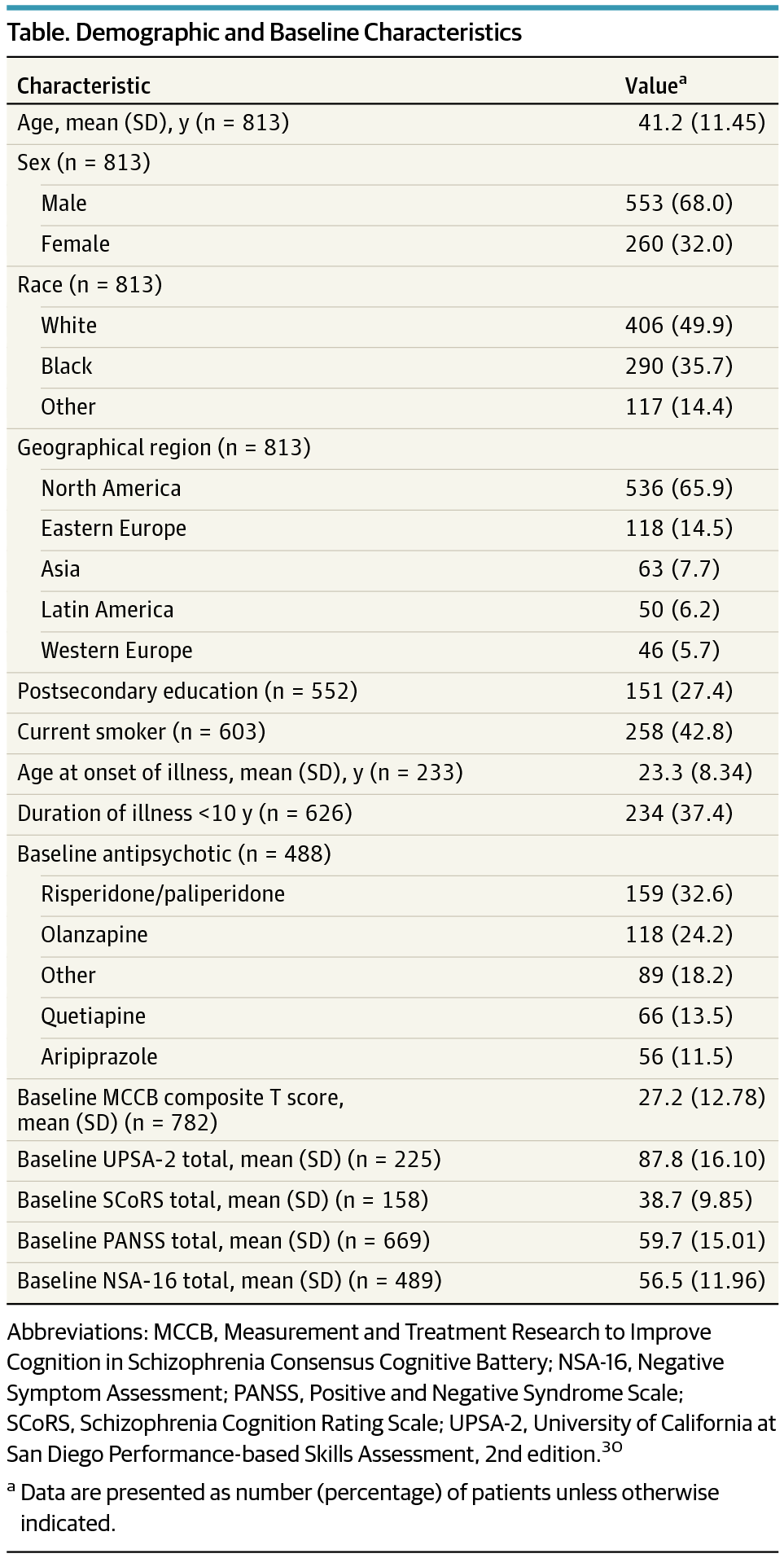
Design, Setting, and Participants A blinded review was conducted of data from 813 patients with schizophrenia who were treated with placebo in 12 randomized placebo-controlled clinical trials conducted mostly in outpatient clinics in North America, Europe, Asia, and Latin America from February 22, 2007, to March 1, 2014. A total of 779 patients provided data for the primary outcome measure at baseline and at least 1 follow-up. Seven trials had prebaseline assessments wherein the patients knew that they were not receiving treatment, allowing a comparison of practice and placebo effects in the same patients.
Interventions Placebo compared with various experimental drug treatments.
Main Outcomes and Measures Composite score on the MCCB.
Results Of the 813 patients in the study (260 women and 553 men; mean [SD] age, 41.2 [11.5] years), the mean MCCB composite score at baseline was 22.8 points below the normative mean, and the mean (SEM) total change in the MCCB during receipt of placebo was 1.8 (0.2) T-score points (95% CI, 1.40-2.18), equivalent to a change of 0.18 SD. Practice effects in the 7 studies in which there was a prebaseline assessment were essentially identical to the postbaseline placebo changes. Baseline factors associated with greater improvements in the MCCB during receipt of placebo included more depression/anxiety (F1,438 = 5.41; P = .02), more motivation (F1,272 = 4.63; P = .03), and less improvement from screening to baseline (F1,421 = 59.32; P < .001).
Conclusions and Relevance Placebo effects were minimal and associated with the number of postbaseline assessments and several patient characteristics. Given that the patients performed 2.28 SDs below normative standards on average at baseline, a mean placebo-associated improvement of less than 0.2 SD provides evidence that ceiling effects do not occur in these trials. These minimal changes in the MCCB could not be responsible for effective active treatments failing to separate from placebo.
中文翻译:

精神分裂症认知试验中的安慰剂反应和实践效果
重要性 患者先前在基于临床表现的认知测试中治疗与精神分裂症相关的认知障碍的经验可以带来与实践相关的改善。与精神分裂症相关的认知障碍的安慰剂对照试验存在这些实践效应的风险,这可能很难与安慰剂效应区分开。
目的 :对测量和治疗研究的实践效果进行系统评估,以改善精神分裂症相关认知障碍的精神分裂症共识认知电池(MCCB)认知,并检查哪些人口统计学,临床和认知特征与改善相关在安慰剂条件下。
设计,背景和参与者 从2月22日开始在12项随机安慰剂对照临床试验(主要在北美,欧洲,亚洲和拉丁美洲的门诊诊所进行)中对来自813名精神分裂症患者的数据进行了盲法审查,这些患者接受了安慰剂治疗。 ,从2007年至2014年3月1日。总共779例患者在基线和至少1次随访中提供了主要结局指标的数据。七项试验进行了基线前评估,其中患者知道他们没有接受治疗,因此可以比较同一患者的实践和安慰剂作用。
干预 安慰剂与各种实验药物治疗的比较。
MCCB的主要成果和衡量指标综合得分。
结果 在本研究的813例患者中(260名女性和553名男性;平均[SD]年龄为41.2 [11.5]岁),基线时的平均MCCB综合评分比标准平均水平低22.8分,且平均水平(SEM)接受安慰剂期间,MCCB的变化为1.8(0.2)T分数(95%CI,1.40-2.18),相当于变化了0.18 SD。在进行基线前评估的7项研究中,实践效果与基线后安慰剂变化基本相同。与接受安慰剂期间MCCB改善更大相关的基线因素包括更多的抑郁/焦虑(F 1,438 = 5.41;P = .02),更多的动机(F 1,272 = 4.63;P = .03),从筛查到基线的改善较小(F 1,421 = 59.32;P <.001)。
结论和相关性 安慰剂的影响极小,并且与基线后评估的次数和一些患者特征有关。假设患者在基线时平均低于规范标准2.28 SD,则安慰剂相关的平均改善低于0.2 SD提供了证据,表明在这些试验中未出现最高限度的影响。MCCB中的这些最小变化不会导致未能从安慰剂中分离出来的有效主动治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号