当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The role of iodide in the formation of lithium hydroxide in lithium–oxygen batteries
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2017-07-17 00:00:00 , DOI: 10.1039/c7ee00954b Michał Tułodziecki 1, 2, 3, 4 , Graham M. Leverick 2, 3, 4, 5 , Chibueze V. Amanchukwu 2, 3, 4, 6 , Yu Katayama 2, 3, 4, 5, 7 , David G. Kwabi 2, 3, 4, 5 , Fanny Bardé 8, 9, 10, 11, 12 , Paula T. Hammond 2, 3, 4, 6 , Yang Shao-Horn 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2017-07-17 00:00:00 , DOI: 10.1039/c7ee00954b Michał Tułodziecki 1, 2, 3, 4 , Graham M. Leverick 2, 3, 4, 5 , Chibueze V. Amanchukwu 2, 3, 4, 6 , Yu Katayama 2, 3, 4, 5, 7 , David G. Kwabi 2, 3, 4, 5 , Fanny Bardé 8, 9, 10, 11, 12 , Paula T. Hammond 2, 3, 4, 6 , Yang Shao-Horn 1, 2, 3, 4, 5
Affiliation
|
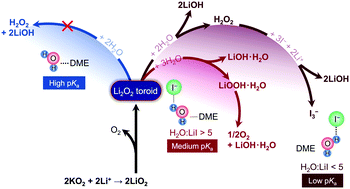
Lithium iodide has been studied extensively as a redox-mediator to reduce the charging overpotential of Li–oxygen (Li–O2) batteries. Ambiguities exist regarding the influence of lithium iodide on the reaction product chemistry and performance of lithium–oxygen batteries. In this work, we examined the role of lithium iodide on the reduction product chemistry under two conditions: (i) mixing KO2 with lithium salts and (ii) discharging Li–oxygen batteries at high and low overpotentials, in the presence of an ether-based electrolyte with different ratios of H2O :
: LiI. The addition of iodide to electrolytes containing water was found to promote the formation of LiOOH·H2O, LiOH·H2O and LiOH at the expense of Li2O2. At low H2O
LiI. The addition of iodide to electrolytes containing water was found to promote the formation of LiOOH·H2O, LiOH·H2O and LiOH at the expense of Li2O2. At low H2O :
: LiI ratios (lower than 5), LiOH instead of Li2O2 was formed, which was accompanied by the oxidation of iodide to triodide while at high H2O
LiI ratios (lower than 5), LiOH instead of Li2O2 was formed, which was accompanied by the oxidation of iodide to triodide while at high H2O :
: LiI ratios (12, 24, 134), a mixture of Li2O2, LiOOH·H2O and LiOH·H2O was observed and no triiodide was detected. The reaction between peroxide Li2O2 and/or superoxide LiO2 with H2O to form LiOH is facilitated by increased water acidity by strong I−–H2O interactions as revealed by 1H NMR and FT-IR measurements. This mechanism of LiOH formation in the presence of LiI and H2O was also found upon Li–O2 cell discharge, which is critical to consider when developing LiI as a redox mediator for Li–O2 batteries.
LiI ratios (12, 24, 134), a mixture of Li2O2, LiOOH·H2O and LiOH·H2O was observed and no triiodide was detected. The reaction between peroxide Li2O2 and/or superoxide LiO2 with H2O to form LiOH is facilitated by increased water acidity by strong I−–H2O interactions as revealed by 1H NMR and FT-IR measurements. This mechanism of LiOH formation in the presence of LiI and H2O was also found upon Li–O2 cell discharge, which is critical to consider when developing LiI as a redox mediator for Li–O2 batteries.
中文翻译:

碘化物在锂氧电池中形成氢氧化锂的作用
碘化锂已被广泛研究作为氧化还原介体,以减少锂氧电池(Li-O 2)的充电过电势。碘化锂对反应产物化学和锂氧电池性能的影响存在歧义。在这项工作中,我们在以下两个条件下研究了碘化锂在还原产物化学上的作用:(i)将KO 2与锂盐混合;(ii)在醚存在下以高和低超电势对锂电池放电H 2 O :
: LiI不同比例的锂基电解质。发现在含水电解质中添加碘化物可促进LiOOH·H 2 O,LiOH·H 2的形成O和LiOH以Li 2 O 2为代价。在低H 2 O
LiI不同比例的锂基电解质。发现在含水电解质中添加碘化物可促进LiOOH·H 2 O,LiOH·H 2的形成O和LiOH以Li 2 O 2为代价。在低H 2 O  :
: LiI比(低于5)下,形成LiOH而不是Li 2 O 2,伴随着碘化物氧化为三碘化物,而在高H 2 O
LiI比(低于5)下,形成LiOH而不是Li 2 O 2,伴随着碘化物氧化为三碘化物,而在高H 2 O  :
: LiI比下(12、24、134 ) ,观察到Li 2 O 2,LiOOH·H 2 O和LiOH·H 2 O的混合物,未检测到三碘化物。过氧化物Li 2 O 2和/或超氧化物LiO 2与H 2的反应1 H NMR和FT-IR测量表明,通过强的I -- H 2 O相互作用增强了水的酸度,从而促进了O形成LiOH的形成。在Li-O 2电池放电后,还发现了在LiI和H 2 O存在下LiOH形成的这种机制,这在将LiI用作Li-O 2电池的氧化还原介体时必须考虑到这一点。
LiI比下(12、24、134 ) ,观察到Li 2 O 2,LiOOH·H 2 O和LiOH·H 2 O的混合物,未检测到三碘化物。过氧化物Li 2 O 2和/或超氧化物LiO 2与H 2的反应1 H NMR和FT-IR测量表明,通过强的I -- H 2 O相互作用增强了水的酸度,从而促进了O形成LiOH的形成。在Li-O 2电池放电后,还发现了在LiI和H 2 O存在下LiOH形成的这种机制,这在将LiI用作Li-O 2电池的氧化还原介体时必须考虑到这一点。
更新日期:2017-08-09
 :
: LiI. The addition of iodide to electrolytes containing water was found to promote the formation of LiOOH·H2O, LiOH·H2O and LiOH at the expense of Li2O2. At low H2O
LiI. The addition of iodide to electrolytes containing water was found to promote the formation of LiOOH·H2O, LiOH·H2O and LiOH at the expense of Li2O2. At low H2O :
: LiI ratios (lower than 5), LiOH instead of Li2O2 was formed, which was accompanied by the oxidation of iodide to triodide while at high H2O
LiI ratios (lower than 5), LiOH instead of Li2O2 was formed, which was accompanied by the oxidation of iodide to triodide while at high H2O :
: LiI ratios (12, 24, 134), a mixture of Li2O2, LiOOH·H2O and LiOH·H2O was observed and no triiodide was detected. The reaction between peroxide Li2O2 and/or superoxide LiO2 with H2O to form LiOH is facilitated by increased water acidity by strong I−–H2O interactions as revealed by 1H NMR and FT-IR measurements. This mechanism of LiOH formation in the presence of LiI and H2O was also found upon Li–O2 cell discharge, which is critical to consider when developing LiI as a redox mediator for Li–O2 batteries.
LiI ratios (12, 24, 134), a mixture of Li2O2, LiOOH·H2O and LiOH·H2O was observed and no triiodide was detected. The reaction between peroxide Li2O2 and/or superoxide LiO2 with H2O to form LiOH is facilitated by increased water acidity by strong I−–H2O interactions as revealed by 1H NMR and FT-IR measurements. This mechanism of LiOH formation in the presence of LiI and H2O was also found upon Li–O2 cell discharge, which is critical to consider when developing LiI as a redox mediator for Li–O2 batteries.
中文翻译:

碘化物在锂氧电池中形成氢氧化锂的作用
碘化锂已被广泛研究作为氧化还原介体,以减少锂氧电池(Li-O 2)的充电过电势。碘化锂对反应产物化学和锂氧电池性能的影响存在歧义。在这项工作中,我们在以下两个条件下研究了碘化锂在还原产物化学上的作用:(i)将KO 2与锂盐混合;(ii)在醚存在下以高和低超电势对锂电池放电H 2 O
 :
: LiI不同比例的锂基电解质。发现在含水电解质中添加碘化物可促进LiOOH·H 2 O,LiOH·H 2的形成O和LiOH以Li 2 O 2为代价。在低H 2 O
LiI不同比例的锂基电解质。发现在含水电解质中添加碘化物可促进LiOOH·H 2 O,LiOH·H 2的形成O和LiOH以Li 2 O 2为代价。在低H 2 O  :
: LiI比(低于5)下,形成LiOH而不是Li 2 O 2,伴随着碘化物氧化为三碘化物,而在高H 2 O
LiI比(低于5)下,形成LiOH而不是Li 2 O 2,伴随着碘化物氧化为三碘化物,而在高H 2 O  :
: LiI比下(12、24、134 ) ,观察到Li 2 O 2,LiOOH·H 2 O和LiOH·H 2 O的混合物,未检测到三碘化物。过氧化物Li 2 O 2和/或超氧化物LiO 2与H 2的反应1 H NMR和FT-IR测量表明,通过强的I -- H 2 O相互作用增强了水的酸度,从而促进了O形成LiOH的形成。在Li-O 2电池放电后,还发现了在LiI和H 2 O存在下LiOH形成的这种机制,这在将LiI用作Li-O 2电池的氧化还原介体时必须考虑到这一点。
LiI比下(12、24、134 ) ,观察到Li 2 O 2,LiOOH·H 2 O和LiOH·H 2 O的混合物,未检测到三碘化物。过氧化物Li 2 O 2和/或超氧化物LiO 2与H 2的反应1 H NMR和FT-IR测量表明,通过强的I -- H 2 O相互作用增强了水的酸度,从而促进了O形成LiOH的形成。在Li-O 2电池放电后,还发现了在LiI和H 2 O存在下LiOH形成的这种机制,这在将LiI用作Li-O 2电池的氧化还原介体时必须考虑到这一点。







































 京公网安备 11010802027423号
京公网安备 11010802027423号