当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of the HIV Attachment Inhibitor BMS-663068. Part 5. Selective C-7 Bromination of the 6-Azaindole Core
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00132 Francisco González-Bobes 1 , Matthew R. Hickey 1 , Benjamin Cohen 1 , Michael Bultman 1 , Ke Chen 1 , Dayne Fanfair 1 , Victor W. Rosso 1 , Neil A. Strotman 1 , Boguslaw Mudryk 1 , Saravanababu Murugesan 1 , Richard L. Schild 1 , Sabrina Ivy 1 , Martin D. Eastgate 1 , Jason T. Sweeney 1 , David A. Conlon 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00132 Francisco González-Bobes 1 , Matthew R. Hickey 1 , Benjamin Cohen 1 , Michael Bultman 1 , Ke Chen 1 , Dayne Fanfair 1 , Victor W. Rosso 1 , Neil A. Strotman 1 , Boguslaw Mudryk 1 , Saravanababu Murugesan 1 , Richard L. Schild 1 , Sabrina Ivy 1 , Martin D. Eastgate 1 , Jason T. Sweeney 1 , David A. Conlon 1
Affiliation
|
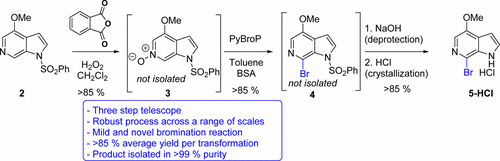
We report research focused on the preparation of an advanced intermediate in the synthesis of a novel antiretroviral. This manuscript describes the development of an efficient oxidation of a 6-azaindole derivative, the bromination of the resulting N-oxide using PyBroP, the removal of the protecting group, and the isolation of the brominated azaindole product. The work reported herein has been successfully implemented in the multikilogram scale to fund development and clinical activities of BMS-663068.
中文翻译:

HIV附着抑制剂BMS-663068的制备。第5部分。6-氮杂吲哚核的选择性C-7溴化反应
我们报告的研究侧重于新型抗逆转录病毒的合成中的高级中间体的制备。该手稿描述了6-氮杂吲哚衍生物的有效氧化,使用PyBroP溴化所得N-氧化物,去除保护基团以及分离溴化氮杂吲哚产物的过程。本文报道的工作已成功以多千克规模实施,以资助BMS-663068的开发和临床活动。
更新日期:2017-08-09
中文翻译:

HIV附着抑制剂BMS-663068的制备。第5部分。6-氮杂吲哚核的选择性C-7溴化反应
我们报告的研究侧重于新型抗逆转录病毒的合成中的高级中间体的制备。该手稿描述了6-氮杂吲哚衍生物的有效氧化,使用PyBroP溴化所得N-氧化物,去除保护基团以及分离溴化氮杂吲哚产物的过程。本文报道的工作已成功以多千克规模实施,以资助BMS-663068的开发和临床活动。











































 京公网安备 11010802027423号
京公网安备 11010802027423号