当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preparation of the HIV Attachment Inhibitor BMS-663068. Part 1. Evolution of Enabling Strategies
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00134 Richard J. Fox 1 , Jonathan C. Tripp 1 , Mitchell J. Schultz 1 , Joseph F. Payack 1 , Dayne D. Fanfair 1 , Boguslaw M. Mudryk 1 , Saravanababu Murugesan 1 , Chung-Pin H. Chen 1 , Thomas E. La Cruz 1 , Sabrina E. Ivy 1 , Sévrine Broxer 1 , Ryan Cullen 1 , Deniz Erdemir 1 , Peng Geng 1 , Zhongmin Xu 1 , Alan Fritz 1 , Wendel W. Doubleday 1 , David A. Conlon 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00134 Richard J. Fox 1 , Jonathan C. Tripp 1 , Mitchell J. Schultz 1 , Joseph F. Payack 1 , Dayne D. Fanfair 1 , Boguslaw M. Mudryk 1 , Saravanababu Murugesan 1 , Chung-Pin H. Chen 1 , Thomas E. La Cruz 1 , Sabrina E. Ivy 1 , Sévrine Broxer 1 , Ryan Cullen 1 , Deniz Erdemir 1 , Peng Geng 1 , Zhongmin Xu 1 , Alan Fritz 1 , Wendel W. Doubleday 1 , David A. Conlon 1
Affiliation
|
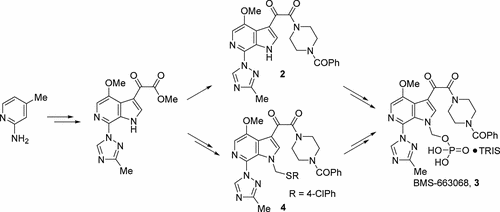
The development of two enabling routes that led to the production of >1000 kg of BMS-663068 (3) is described. The route identified for the initial 100 kg delivery to support development activities and initial clinical trials involved the conversion of 2-amino-4-picoline to the parent active pharmaceutical ingredient (API), followed by pro-drug installation and deprotection. To eliminate the problematic isolation of the parent API and synthesis of di-t-butyl(chloromethyl)phosphate, a second-generation pro-drug installation route was developed which involved the conversion of a late-stage common intermediate to an N(1)-thioether derivative followed by chloromethylation, displacement with di-t-butylpotassium phosphate, and deprotection. This second strategy resulted in the multikilogram scale preparation of the API in 14 linear steps and ∼7% overall yield.
中文翻译:

HIV附着抑制剂BMS-663068的制备。第1部分。授权策略的演变
描述了开发两个导致生产> 1000 kg BMS-663068(3)的可行途径的过程。为支持发展活动和最初的临床试验而确定的最初100千克递送的路线涉及将2-氨基-4-甲基吡啶转化为母体活性药物成分(API),然后进行前药安装和脱保护。为了消除二的父API和合成的问题的隔离吨丁基(氯甲基)酯,磷酸一第二代前药安装路线被开发其中涉及一个后期常用中间体的转化为N(1) -thioether衍生物随后氯甲基,位移二吨丁基磷酸钾,并脱保护。第二种策略导致API以14线性步长的多千克级规模制备API,总产率约为7%。
更新日期:2017-08-09
中文翻译:

HIV附着抑制剂BMS-663068的制备。第1部分。授权策略的演变
描述了开发两个导致生产> 1000 kg BMS-663068(3)的可行途径的过程。为支持发展活动和最初的临床试验而确定的最初100千克递送的路线涉及将2-氨基-4-甲基吡啶转化为母体活性药物成分(API),然后进行前药安装和脱保护。为了消除二的父API和合成的问题的隔离吨丁基(氯甲基)酯,磷酸一第二代前药安装路线被开发其中涉及一个后期常用中间体的转化为N(1) -thioether衍生物随后氯甲基,位移二吨丁基磷酸钾,并脱保护。第二种策略导致API以14线性步长的多千克级规模制备API,总产率约为7%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号