当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of the HIV Attachment Inhibitor BMS-663068. Part 9. Active Pharmaceutical Ingredient Process Development and Powder Properties
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00138 Thomas E. La Cruz 1 , Eric M. Saurer 1 , Joshua Engstrom 1 , Michael S. Bultman 1 , Robert Forest 1 , Fulya Akpinar 1 , Glenn Ferreira 1 , Jeanne W. Ho 1 , Masano Huang 1 , Michelle Soltani 1 , Saravanababu Murugesan 1 , Dayne Fanfair 1 , Antonio Ramirez 1 , Victor W. Rosso 1 , Deniz Erdemir 1 , Tamar Rosenbaum 1 , Michelle Haslam 1 , Stephen Grier 1 , Michael Peddicord 1 , Charles Pathirana 1 , Jonathan Marshall 1 , Wei Ding 1 , Yande Huang 1 , Sloan Ayers 1 , Alan Braem 1 , Richard L. Schild 1 , Sabrina E. Ivy 1 , Joseph Payack 1 , Douglas D. McLeod 1 , Whitney Nikitczuk 1 , Wendel Doubleday 1 , Sapna Shah 1 , David A. Conlon 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00138 Thomas E. La Cruz 1 , Eric M. Saurer 1 , Joshua Engstrom 1 , Michael S. Bultman 1 , Robert Forest 1 , Fulya Akpinar 1 , Glenn Ferreira 1 , Jeanne W. Ho 1 , Masano Huang 1 , Michelle Soltani 1 , Saravanababu Murugesan 1 , Dayne Fanfair 1 , Antonio Ramirez 1 , Victor W. Rosso 1 , Deniz Erdemir 1 , Tamar Rosenbaum 1 , Michelle Haslam 1 , Stephen Grier 1 , Michael Peddicord 1 , Charles Pathirana 1 , Jonathan Marshall 1 , Wei Ding 1 , Yande Huang 1 , Sloan Ayers 1 , Alan Braem 1 , Richard L. Schild 1 , Sabrina E. Ivy 1 , Joseph Payack 1 , Douglas D. McLeod 1 , Whitney Nikitczuk 1 , Wendel Doubleday 1 , Sapna Shah 1 , David A. Conlon 1
Affiliation
|
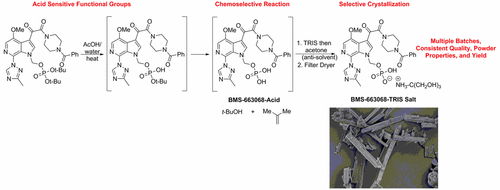
This final communication, of a nine part publication series, details the process development history for the final synthetic step to prepare the drug substance BMS-663068 tris(hydroxymethyl)aminomethane (TRIS) salt. The challenge of developing a robust commercial process to prepare BMS-663068-TRIS salt (active pharmaceutical ingredient, API) was achieved by studying the underlying mechanisms that governed key processing characteristics. Eliminating a slurry-to-slurry transformation results in predictable reaction kinetics and control of impurity formation. Key powder property aspects, such as specific surface area and bulk density, were controlled by examining the impact of seed age, crystallization relative supersaturation (RSS), and particle attrition due to agitation during drying. Ultimately, the processing parameters established for preparation of this drug substance resulted in the generation of the target compound with consistent quality, powder properties, and yield across multiple batches.
中文翻译:

HIV附着抑制剂BMS-663068的制备。第9部分。活性药物成分的工艺开发和粉末性质
这是一本由9个部分组成的出版物系列的最终交流,详细介绍了制备药物BMS-663068三(羟甲基)氨基甲烷(TRIS)盐的最终合成步骤的工艺开发历史。通过研究控制关键加工特征的潜在机制,解决了开发强大的商业流程以制备BMS-663068-TRIS盐(活性药物成分,API)的挑战。消除浆液到浆液的转化可实现可预测的反应动力学并控制杂质的形成。通过检查种子年龄,结晶相对过饱和度(RSS)和干燥过程中搅拌引起的颗粒磨损的影响,可以控制粉末的关键性能方面,例如比表面积和堆积密度。最终,
更新日期:2017-08-09
中文翻译:

HIV附着抑制剂BMS-663068的制备。第9部分。活性药物成分的工艺开发和粉末性质
这是一本由9个部分组成的出版物系列的最终交流,详细介绍了制备药物BMS-663068三(羟甲基)氨基甲烷(TRIS)盐的最终合成步骤的工艺开发历史。通过研究控制关键加工特征的潜在机制,解决了开发强大的商业流程以制备BMS-663068-TRIS盐(活性药物成分,API)的挑战。消除浆液到浆液的转化可实现可预测的反应动力学并控制杂质的形成。通过检查种子年龄,结晶相对过饱和度(RSS)和干燥过程中搅拌引起的颗粒磨损的影响,可以控制粉末的关键性能方面,例如比表面积和堆积密度。最终,











































 京公网安备 11010802027423号
京公网安备 11010802027423号