当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
(Chloromethyl)dimethylchlorosilane–KF: A Two-Step Solution to the Selectivity Problem in the Methylation of a Pyrimidone Intermediate en Route to Raltegravir
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1021/acs.oprd.7b00171 Christos I. Stathakis 1 , Petros L. Gkizis 1 , Elli S. Alexandraki 1 , Sakellarios Trakossas 1 , Michael Terzidis 1 , Efstratios Neokosmidis 1 , Constantinos K. Zacharis 1 , Christina Vasiliadou 1 , Elli Vastardi 1 , Thanos Andreou 1 , Asteria Zitrou 1 , Anastasia-Aikaterini Varvogli 1 , Theocharis V. Koftis 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1021/acs.oprd.7b00171 Christos I. Stathakis 1 , Petros L. Gkizis 1 , Elli S. Alexandraki 1 , Sakellarios Trakossas 1 , Michael Terzidis 1 , Efstratios Neokosmidis 1 , Constantinos K. Zacharis 1 , Christina Vasiliadou 1 , Elli Vastardi 1 , Thanos Andreou 1 , Asteria Zitrou 1 , Anastasia-Aikaterini Varvogli 1 , Theocharis V. Koftis 1
Affiliation
|
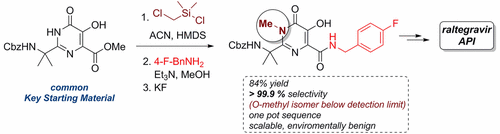
The present work describes a two-step process, namely, silylation with (chloromethyl)dimethylchlorosilane and desilylation, to address the selectivity problem in the N-methylation of a pyrimidone intermediate toward the synthesis of the raltegravir active pharmaceutical ingredient. The said methodology delivers the desired drug substance in which the O-methylated impurity content is below the detection limit by high-performance liquid chromatography analysis. Moreover, this two-step, one-pot procedure provides an apparent advantage in terms of environmental impact with respect to the optimum approach described in the literature, while it compares equally well in terms of cost and operational simplicity.
中文翻译:

(氯甲基)二甲基氯硅烷-KF:两步解决嘧啶酮中间体向Raltegravir甲基化的选择性问题
本工作描述了两步法,即用(氯甲基)二甲基氯硅烷甲硅烷基化和去甲硅烷基化,以解决嘧啶酮中间体在N-甲基化过程中对合成raltegravir活性药物成分的选择性问题。所述方法提供了所需的药物,其中通过高效液相色谱分析,O-甲基化的杂质含量低于检测极限。而且,相对于文献中描述的最佳方法,这种两步一锅法在环境影响方面提供了明显的优势,同时在成本和操作简便性方面也具有相当的优势。
更新日期:2017-08-07
中文翻译:

(氯甲基)二甲基氯硅烷-KF:两步解决嘧啶酮中间体向Raltegravir甲基化的选择性问题
本工作描述了两步法,即用(氯甲基)二甲基氯硅烷甲硅烷基化和去甲硅烷基化,以解决嘧啶酮中间体在N-甲基化过程中对合成raltegravir活性药物成分的选择性问题。所述方法提供了所需的药物,其中通过高效液相色谱分析,O-甲基化的杂质含量低于检测极限。而且,相对于文献中描述的最佳方法,这种两步一锅法在环境影响方面提供了明显的优势,同时在成本和操作简便性方面也具有相当的优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号