当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Integrin‐Mediated Interactions Control Macrophage Polarization in 3D Hydrogels
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2017-08-07 , DOI: 10.1002/adhm.201700289 Byung-Hyun Cha 1, 2, 3 , Su Ryon Shin 1, 2, 3 , Jeroen Leijten 1, 2, 4 , Yi-Chen Li 1, 2, 3 , Sonali Singh 1, 2, 5 , Julie C Liu 1, 2, 6 , Nasim Annabi 1, 2, 7 , Reza Abdi 1, 2, 8 , Mehmet R Dokmeci 1, 2, 3 , Nihal Engin Vrana 1, 2, 9, 10 , Amir M Ghaemmaghami 1, 2, 5 , Ali Khademhosseini 1, 2, 3, 11, 12
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2017-08-07 , DOI: 10.1002/adhm.201700289 Byung-Hyun Cha 1, 2, 3 , Su Ryon Shin 1, 2, 3 , Jeroen Leijten 1, 2, 4 , Yi-Chen Li 1, 2, 3 , Sonali Singh 1, 2, 5 , Julie C Liu 1, 2, 6 , Nasim Annabi 1, 2, 7 , Reza Abdi 1, 2, 8 , Mehmet R Dokmeci 1, 2, 3 , Nihal Engin Vrana 1, 2, 9, 10 , Amir M Ghaemmaghami 1, 2, 5 , Ali Khademhosseini 1, 2, 3, 11, 12
Affiliation
|
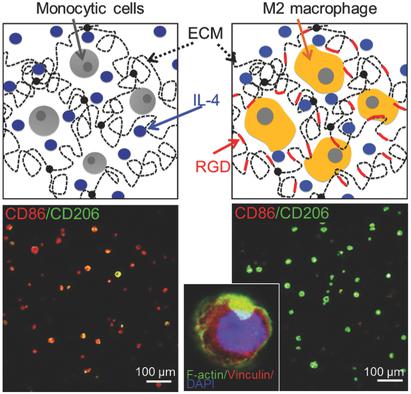
Adverse immune reactions prevent clinical translation of numerous implantable devices and materials. Although inflammation is an essential part of tissue regeneration, chronic inflammation ultimately leads to implant failure. In particular, macrophage polarity steers the microenvironment toward inflammation or wound healing via the induction of M1 and M2 macrophages, respectively. Here, this paper demonstrates that macrophage polarity within biomaterials can be controlled through integrin‐mediated interactions between human monocytic THP‐1 cells and collagen‐derived matrix. Surface marker, gene expression, biochemical, and cytokine profiling consistently indicate that THP‐1 cells within a biomaterial lacking cell attachment motifs yield proinflammatory M1 macrophages, whereas biomaterials with attachment sites in the presence of interleukin‐4 (IL‐4) induce an anti‐inflammatory M2‐like phenotype and propagate the effect of IL‐4 in induction of M2‐like macrophages. Importantly, integrin α2β1 plays a pivotal role as its inhibition blocks the induction of M2 macrophages. The influence of the microenvironment of the biomaterial over macrophage polarity is further confirmed by its ability to modulate the effect of IL‐4 and lipopolysaccharide, which are potent inducers of M2 or M1 phenotypes, respectively. Thus, this study represents a novel, versatile, and effective strategy to steer macrophage polarity through integrin‐mediated 3D microenvironment for biomaterial‐based programming.
中文翻译:

整合素介导的相互作用控制 3D 水凝胶中的巨噬细胞极化
不良免疫反应阻碍了许多可植入设备和材料的临床转化。尽管炎症是组织再生的重要组成部分,但慢性炎症最终会导致植入失败。特别是,巨噬细胞极性分别通过诱导 M1 和 M2 巨噬细胞将微环境引导至炎症或伤口愈合。本文证明,生物材料内的巨噬细胞极性可以通过整合素介导的人类单核 THP-1 细胞和胶原衍生基质之间的相互作用来控制。表面标记、基因表达、生化和细胞因子分析一致表明,缺乏细胞附着基序的生物材料内的 THP-1 细胞会产生促炎性 M1 巨噬细胞,而在存在白细胞介素-4 (IL-4) 的情况下,具有附着位点的生物材料会诱导抗炎症反应。 ‐炎症 M2 样表型并传播 IL-4 在诱导 M2 样巨噬细胞中的作用。重要的是,整合素 α2β1 发挥着关键作用,因为它的抑制作用会阻止 M2 巨噬细胞的诱导。生物材料的微环境对巨噬细胞极性的影响通过其调节 IL-4 和脂多糖作用的能力得到进一步证实,IL-4 和脂多糖分别是 M2 或 M1 表型的有效诱导剂。因此,这项研究代表了一种新颖、通用且有效的策略,通过整合素介导的 3D 微环境来控制巨噬细胞极性,以进行基于生物材料的编程。
更新日期:2017-08-07
中文翻译:

整合素介导的相互作用控制 3D 水凝胶中的巨噬细胞极化
不良免疫反应阻碍了许多可植入设备和材料的临床转化。尽管炎症是组织再生的重要组成部分,但慢性炎症最终会导致植入失败。特别是,巨噬细胞极性分别通过诱导 M1 和 M2 巨噬细胞将微环境引导至炎症或伤口愈合。本文证明,生物材料内的巨噬细胞极性可以通过整合素介导的人类单核 THP-1 细胞和胶原衍生基质之间的相互作用来控制。表面标记、基因表达、生化和细胞因子分析一致表明,缺乏细胞附着基序的生物材料内的 THP-1 细胞会产生促炎性 M1 巨噬细胞,而在存在白细胞介素-4 (IL-4) 的情况下,具有附着位点的生物材料会诱导抗炎症反应。 ‐炎症 M2 样表型并传播 IL-4 在诱导 M2 样巨噬细胞中的作用。重要的是,整合素 α2β1 发挥着关键作用,因为它的抑制作用会阻止 M2 巨噬细胞的诱导。生物材料的微环境对巨噬细胞极性的影响通过其调节 IL-4 和脂多糖作用的能力得到进一步证实,IL-4 和脂多糖分别是 M2 或 M1 表型的有效诱导剂。因此,这项研究代表了一种新颖、通用且有效的策略,通过整合素介导的 3D 微环境来控制巨噬细胞极性,以进行基于生物材料的编程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号