Journal of Controlled Release ( IF 10.5 ) Pub Date : 2017-08-05 , DOI: 10.1016/j.jconrel.2017.08.005 Zheng Chai 1 , Junjiang Sun 1 , Kelly Michelle Rigsbee 1 , Mei Wang 2 , R Jude Samulski 3 , Chengwen Li 4
|
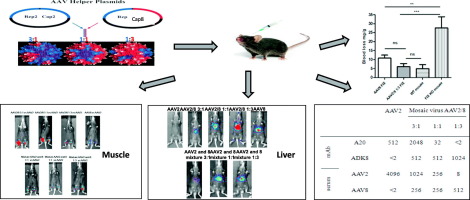
Adeno-associated virus (AAV) vectors have been used successfully in clinical trials for patients with hemophilia or blindness, but pre-existing neutralizing antibodies (Nab) are common in the general population and exclude many patients from clinical trials. Exploration of effective strategies to enhance AAV transduction and escape from Nab activity is still imperative. Previous studies have shown the compatibility of capsids from AAV serotypes and homology of recognition sites of AAV Nab located on different capsid subunits from one virion. In this study, we co-transfected AAV2 and AAV8 helper plasmids at different ratios (3:1, 1:1 and 1:3) to assemble haploid capsids and study both their transduction efficiency and Nab escape activity. After muscular injection, all of the haploid viruses induced higher transduction than their parental AAV vectors (2- to 9-fold over AAV2), with the highest of these being the haploid vector AAV2/8 3:1. After systemic administration, a 4-fold higher transduction in the liver was observed with haploid AAV2/8 1:3 than that with AAV8 alone. We then packaged the therapeutic factor IX cassette into haploid AAV2/8 1:3 capsids and injected them into FIX knockout mice via the tail vein. Higher FIX expression and improved phenotypic correction were achieved with the haploid AAV2/8 1:3 virus vector when compared to that of AAV8. Additionally, the haploid virus AAV2/8 1:3 was able to escape AAV2 neutralization and did not increase capsid antigen presentation capacity when compared to AAV8. To improve the Nab evasion ability of the haploid virus, we produced the triploid vector AAV2/8/9 by co-transfecting AAV2, AAV8 and AAV9 helper plasmids at a ratio of 1:1:1. After systemic administration, a 2-fold higher transduction in the liver was observed with the triploid vector AAV2/8/9 than that with AAV8. Nab analysis demonstrated that the triploid AAV2/8/9 vector was able to escape Nab activity from mouse sera immunized with parental serotypes. These results indicate that polyploid viruses might potentially acquire advantages from parental serotypes for enhancement of AAV transduction and evasion of Nab recognition without increasing capsid antigen presentation in target cells. Polyploid AAV vectors can be generated from any AAV serotype, whether natural, rational, library derived or a combination thereof, providing a novel strategy that should be explored in future clinical trials in patients with neutralizing antibodies.
中文翻译:

多倍体腺相关病毒载体在转导增强和中和抗体逃避中的应用
腺相关病毒(AAV)载体已成功用于血友病或失明患者的临床试验,但预先存在的中和抗体(Nab)在普通人群中很常见,因此将许多患者排除在临床试验之外。探索增强 AAV 转导和逃避 Nab 活性的有效策略仍然势在必行。先前的研究表明,AAV 血清型衣壳的兼容性以及位于一种病毒体不同衣壳亚基上的 AAV Nab 识别位点的同源性。在本研究中,我们以不同比例(3:1、1:1 和 1:3)共转染 AAV2 和 AAV8 辅助质粒来组装单倍体衣壳,并研究其转导效率和 Nab 逃逸活性。肌肉注射后,所有单倍体病毒诱导的转导率均高于其亲本 AAV 载体(是 AAV2 的 2 至 9 倍),其中最高的是单倍体载体 AAV2/8 3:1。全身给药后,观察到单倍体 AAV2/8 1:3 的肝脏转导比单独使用 AAV8 高 4 倍。然后,我们将治疗因子 IX 盒包装到单倍体 AAV2/8 1:3 衣壳中,并通过尾静脉将它们注射到 FIX 敲除小鼠体内。与 AAV8 相比,单倍体 AAV2/8 1:3 病毒载体实现了更高的 FIX 表达和改进的表型校正。此外,与 AAV8 相比,单倍体病毒 AAV2/8 1:3 能够逃避 AAV2 中和,并且不会增加衣壳抗原呈递能力。为了提高单倍体病毒的Nab逃避能力,我们通过以1:1:1的比例共转染AAV2、AAV8和AAV9辅助质粒来产生三倍体载体AAV2/8/9。 全身给药后,观察到三倍体载体AAV2/8/9在肝脏中的转导率比AAV8高2倍。 Nab 分析表明,三倍体 AAV2/8/9 载体能够逃避用亲本血清型免疫的小鼠血清中的 Nab 活性。这些结果表明,多倍体病毒可能从亲本血清型中获得优势,以增强 AAV 转导和逃避 Nab 识别,而不增加靶细胞中衣壳抗原的呈递。多倍体 AAV 载体可以从任何 AAV 血清型产生,无论是天然的、合理的、文库衍生的还是它们的组合,这提供了一种新的策略,应该在未来针对具有中和抗体的患者的临床试验中进行探索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号