当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Kinetics of CaOH with H and O2 and O2CaOH with O: Implications for the Atmospheric Chemistry of Meteoric Calcium
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2017-08-04 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00072 Juan Carlos Gomez Martin 1 , John M. C. Plane 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2017-08-04 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00072 Juan Carlos Gomez Martin 1 , John M. C. Plane 1
Affiliation
|
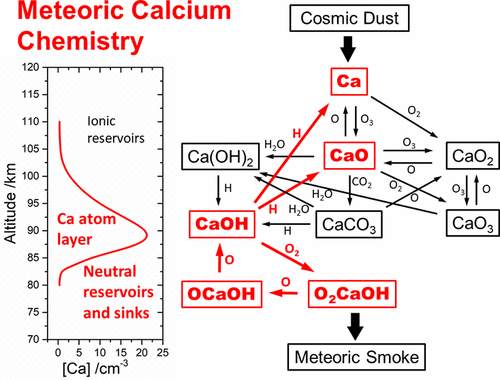
The ablation of cosmic dust particles entering the Earth’s upper atmosphere produces a layer of Ca atoms around 90 km. Here, we present a set of kinetic experiments designed to understand the nature of the Ca molecular reservoirs on the underside of the layer. CaOH was produced by laser ablation of a Ca target in the fast flow tube and detected by non-resonant laser-induced fluorescence, probing the D(2Σ+) ← X(2Σ1) transition at 346.9 nm. The following rate constants were measured (at 298 K): k(CaOH + H → Ca + H2O) = (1.04 ± 0.24) × 10–10 cm3 molecule–1 s–1, k(CaOH + O → CaO + OH) < 1 × 10–11 cm3 molecule–1 s–1, and k(CaOH + O2 → O2CaOH, 1 Torr) = (5.9 ± 1.8) × 10–11 cm3 molecule–1 s–1 (uncertainty at the 2σ level of confidence). The recycling of CaOH from reaction between O2CaOH and O proceeds with an effective rate constant of keff(O2CaOH + O → CaOH + products, 298 K) = 2.8–1.2+2.0 × 10–10 cm3 molecule–1 s–1. Master equation modeling of the CaOH + O2 kinetics is used to extrapolate to mesospheric temperatures and pressures. The results suggest that the formation of O2CaOH slows the conversion of CaOH to atomic Ca via reaction with atomic H, and O2CaOH is likely to be a long-lived reservoir species on the underside of the Ca layer and a building block of meteoric smoke particles.
中文翻译:

CaOH与H和O 2的反应动力学以及O 2 CaOH与O的反应动力学:对流钙的大气化学意义
进入地球高层大气的宇宙尘埃粒子的烧蚀在90公里处产生一层Ca原子。在这里,我们提出了一组动力学实验,旨在了解该层下层的Ca分子储层的性质。CaOH中通过在快速流管一钙靶的激光烧蚀产生并由非谐振的激光诱导的荧光检测,探测d(2 Σ +)←X(2 Σ 1)过渡在346.9纳米。测量了以下速率常数(在298 K下):k(CaOH + H→Ca + H 2 O)=(1.04±0.24)×10 –10 cm 3分子–1 s –1,k(CaOH + O→CaO + OH)<1×10 –11 cm 3分子–1 s –1和k(CaOH + O 2 →O 2 CaOH,1 Torr)=(5.9±1.8)×10 –11 cm 3个分子–1 s –1(在2σ置信水平下的不确定性)。从O 2 CaOH和O之间的反应中回收CaOH的有效速率常数为k eff(O 2 CaOH + O→CaOH +产物,298 K)= 2.8 –1.2 +2.0 ×10 –10 cm 3分子–1s –1。CaOH + O 2动力学的主方程模型用于推断中层温度和压力。结果表明,O 2 CaOH的形成通过与原子H的反应减慢了CaOH向原子Ca的转化,O 2 CaOH可能是Ca层下侧的长寿储层物质,是Ca的构造单元。巨大的烟雾颗粒。
更新日期:2017-08-04
中文翻译:

CaOH与H和O 2的反应动力学以及O 2 CaOH与O的反应动力学:对流钙的大气化学意义
进入地球高层大气的宇宙尘埃粒子的烧蚀在90公里处产生一层Ca原子。在这里,我们提出了一组动力学实验,旨在了解该层下层的Ca分子储层的性质。CaOH中通过在快速流管一钙靶的激光烧蚀产生并由非谐振的激光诱导的荧光检测,探测d(2 Σ +)←X(2 Σ 1)过渡在346.9纳米。测量了以下速率常数(在298 K下):k(CaOH + H→Ca + H 2 O)=(1.04±0.24)×10 –10 cm 3分子–1 s –1,k(CaOH + O→CaO + OH)<1×10 –11 cm 3分子–1 s –1和k(CaOH + O 2 →O 2 CaOH,1 Torr)=(5.9±1.8)×10 –11 cm 3个分子–1 s –1(在2σ置信水平下的不确定性)。从O 2 CaOH和O之间的反应中回收CaOH的有效速率常数为k eff(O 2 CaOH + O→CaOH +产物,298 K)= 2.8 –1.2 +2.0 ×10 –10 cm 3分子–1s –1。CaOH + O 2动力学的主方程模型用于推断中层温度和压力。结果表明,O 2 CaOH的形成通过与原子H的反应减慢了CaOH向原子Ca的转化,O 2 CaOH可能是Ca层下侧的长寿储层物质,是Ca的构造单元。巨大的烟雾颗粒。











































 京公网安备 11010802027423号
京公网安备 11010802027423号