PLOS Biology ( IF 7.8 ) Pub Date : 2017-08-03 , DOI: 10.1371/journal.pbio.2002281 Takuma Fukumura , Fumiaki Makino , Tobias Dietsche , Miki Kinoshita , Takayuki Kato , Samuel Wagner , Keiichi Namba , Katsumi Imada , Tohru Minamino
|
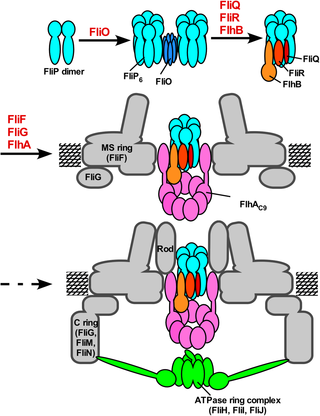
The bacterial flagellar type III export apparatus, which is required for flagellar assembly beyond the cell membranes, consists of a transmembrane export gate complex and a cytoplasmic ATPase complex. FlhA, FlhB, FliP, FliQ, and FliR form the gate complex inside the basal body MS ring, although FliO is required for efficient export gate formation in Salmonella enterica. However, it remains unknown how they form the gate complex. Here we report that FliP forms a homohexameric ring with a diameter of 10 nm. Alanine substitutions of conserved Phe-137, Phe-150, and Glu-178 residues in the periplasmic domain of FliP (FliPP) inhibited FliP6 ring formation, suppressing flagellar protein export. FliO formed a 5-nm ring structure with 3 clamp-like structures that bind to the FliP6 ring. The crystal structure of FliPP derived from Thermotoga maritia, and structure-based photo-crosslinking experiments revealed that Phe-150 and Ser-156 of FliPP are involved in the FliP–FliP interactions and that Phe-150, Arg-152, Ser-156, and Pro-158 are responsible for the FliP–FliO interactions. Overexpression of FliP restored motility of a ∆fliO mutant to the wild-type level, suggesting that the FliP6 ring is a functional unit in the export gate complex and that FliO is not part of the final gate structure. Copurification assays revealed that FlhA, FlhB, FliQ, and FliR are associated with the FliO/FliP complex. We propose that the assembly of the export gate complex begins with FliP6 ring formation with the help of the FliO scaffold, followed by FliQ, FliR, and FlhB and finally FlhA during MS ring formation.
中文翻译:

组装和化学计量的细菌鞭毛III型出口门复合体的核心结构。
鞭毛组装到细胞膜外所必需的细菌鞭毛III型输出设备由跨膜输出门复合物和细胞质ATPase复合物组成。FlhA,FlhB,FliP,FliQ和FliR在基体MS环内部形成了门复合物,尽管在肠沙门氏菌中高效形成出口门也需要FliO 。然而,仍然不清楚它们如何形成门复合体。在这里,我们报道FliP形成直径为10 nm的同六聚环。FliP(FliP P)的周质结构域中保守的Phe-137,Phe-150和Glu-178残基的丙氨酸替代抑制FliP 6环形成,抑制鞭毛蛋白输出。FliO形成了一个具有3个与FliP 6环结合的钳状结构的5 nm环结构。源自海栖嗜热菌的FliP P的晶体结构以及基于结构的光交联实验表明,FliP P的Phe-150和Ser-156与FliP–FliP相互作用有关,而Phe-150,Arg-152,Ser -156和Pro-158负责FliP–FliO交互。FLIP的过表达恢复了Δ的蠕动fliO突变体与野生型的水平,这表明该倒装6环是出口门综合体中的功能单元,FliO不是最终门结构的一部分。共纯化测定显示FlhA,FlhB,FliQ和FliR与FliO / FliP复合物相关。我们建议在FliO支架的帮助下开始组装FliP 6环,然后在MS环形成过程中首先进行FliQ,FliR和FlhB,最后是FlhA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号