当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel Strategy Utilizing Extracellular Cysteine-Rich Domain of Membrane Receptor for Constructing d-Peptide Mediated Targeted Drug Delivery Systems: A Case Study on Fn14
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-08-02 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00326 Zhuoxuan Li 1 , Jing Xie 1 , Shan Peng 1 , Sha Liu 1 , Ying Wang 1 , Weiyue Lu 2 , Jie Shen 3 , Chong Li 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-08-02 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00326 Zhuoxuan Li 1 , Jing Xie 1 , Shan Peng 1 , Sha Liu 1 , Ying Wang 1 , Weiyue Lu 2 , Jie Shen 3 , Chong Li 1
Affiliation
|
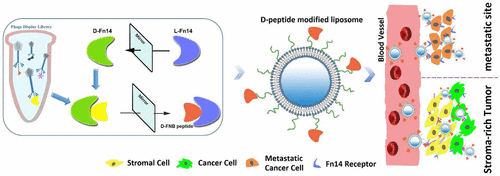
The development of proteolysis-resistant d-peptide ligands for targeted drug/gene delivery has been greatly limited, due to the challenge that lies in the chemical synthesis of membrane receptors without altering their structures. In the present research, a novel strategy utilizing self-stabilized extracellular CRD of the membrane receptor was developed to construct d-peptide ligands and their mediated targeted drug delivery systems. Fn14, a cell surface receptor overexpressed in many cancers including pancreatic and triple-negative breast cancers, was selected as the model receptor. Fn14 CRD was synthesized and folded, and used to screen Fn14 binding peptides using phage display (l-peptide) and mirror-image phage display (d-peptide) techniques, respectively. The d-peptide ligand successfully mediated targeted drug delivery to Fn14 positive tumor cells. In addition, the d-peptide possessed better target-binding affinity, stromal barrier permeability, and tumor targeting ability in vivo when conjugated with liposomes. More importantly, d-peptide mediated liposomal paclitaxel delivery significantly inhibited pancreatic tumor growth in a subcutaneous xenograft model and drastically prolonged survival in a lung metastasis of breast cancer mouse model. This study demonstrated that mirror-image phage display based on the CRD of membrane receptor can be a promising strategy to advance active targeted drug delivery via biostable d-peptides.
中文翻译:

利用细胞外半胱氨酸丰富的膜受体域构建d肽介导的靶向药物递送系统的新策略:以Fn14为例
由于在膜受体的化学合成而不改变其结构的挑战下,用于靶向药物/基因递送的耐蛋白水解的d-肽配体的开发受到极大的限制。在本研究中,开发了利用膜受体自稳定的细胞外CRD的新策略来构建d肽配体及其介导的靶向药物递送系统。Fn14是一种在许多癌症(包括胰腺癌和三阴性乳腺癌)中过表达的细胞表面受体,被选作模型受体。合成并折叠Fn14 CRD,并用于通过噬菌体展示(l-肽)和镜像噬菌体展示(d-肽)技术。的d -肽配体介导的成功靶向药物递送至FN14阳性肿瘤细胞。另外,当与脂质体缀合时,d-肽在体内具有更好的靶结合亲和力,基质屏障通透性和肿瘤靶向能力。更重要的是,在皮下异种移植模型中,d肽介导的紫杉醇脂质体递送显着抑制了胰腺肿瘤的生长,并在乳腺癌小鼠模型的肺转移中显着延长了生存期。这项研究表明,基于膜受体的CRD镜像噬菌体展示可以以推进经由生物稳定的活性靶向药物递送有希望的策略d-肽。
更新日期:2017-08-03
中文翻译:

利用细胞外半胱氨酸丰富的膜受体域构建d肽介导的靶向药物递送系统的新策略:以Fn14为例
由于在膜受体的化学合成而不改变其结构的挑战下,用于靶向药物/基因递送的耐蛋白水解的d-肽配体的开发受到极大的限制。在本研究中,开发了利用膜受体自稳定的细胞外CRD的新策略来构建d肽配体及其介导的靶向药物递送系统。Fn14是一种在许多癌症(包括胰腺癌和三阴性乳腺癌)中过表达的细胞表面受体,被选作模型受体。合成并折叠Fn14 CRD,并用于通过噬菌体展示(l-肽)和镜像噬菌体展示(d-肽)技术。的d -肽配体介导的成功靶向药物递送至FN14阳性肿瘤细胞。另外,当与脂质体缀合时,d-肽在体内具有更好的靶结合亲和力,基质屏障通透性和肿瘤靶向能力。更重要的是,在皮下异种移植模型中,d肽介导的紫杉醇脂质体递送显着抑制了胰腺肿瘤的生长,并在乳腺癌小鼠模型的肺转移中显着延长了生存期。这项研究表明,基于膜受体的CRD镜像噬菌体展示可以以推进经由生物稳定的活性靶向药物递送有希望的策略d-肽。











































 京公网安备 11010802027423号
京公网安备 11010802027423号