当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Total Synthesis of (−)-Astakolactin and Confirmation of Its Stereostructure
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-08-02 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00368 Takayuki Tonoi 1 , Yutaka Yoshinaga 1 , Moe Fujishiro 1 , Keisuke Mameda 1 , Takashi Kato 1 , Kentaro Shibamoto 1 , Isamu Shiina 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-08-02 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00368 Takayuki Tonoi 1 , Yutaka Yoshinaga 1 , Moe Fujishiro 1 , Keisuke Mameda 1 , Takashi Kato 1 , Kentaro Shibamoto 1 , Isamu Shiina 1
Affiliation
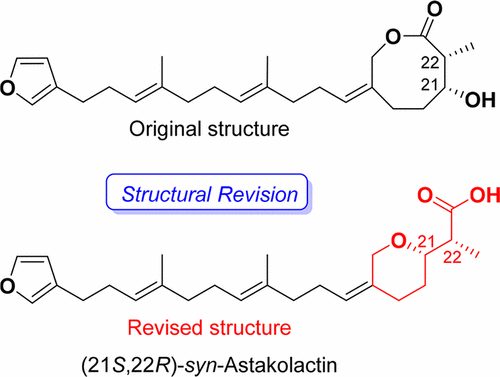
|
The originally proposed structure of astakolactin was revised, and an asymmetric total synthesis of the newly proposed structure was achieved. The key transformations in the synthesis were a Johnson–Claisen rearrangement, an asymmetric Mukaiyama aldol reaction, and a Mitsunobu-type cyclodehydration. The spectroscopic data and specific rotation of the compound obtained matched well with those reported for naturally occurring astakolactin.
中文翻译:

(-)-Astakolactin的不对称全合成及其立体结构的确认
修改了最初提出的黄体泌乳素的结构,并实现了新提出结构的不对称全合成。合成过程中的关键转变是Johnson-Claisen重排,不对称的Mukaiyama aldol反应和Mitsunobu型环脱水。所获得化合物的光谱数据和比旋光度与报道的天然存在的astakolactin的光谱数据和比旋光度非常匹配。
更新日期:2017-08-02
中文翻译:

(-)-Astakolactin的不对称全合成及其立体结构的确认
修改了最初提出的黄体泌乳素的结构,并实现了新提出结构的不对称全合成。合成过程中的关键转变是Johnson-Claisen重排,不对称的Mukaiyama aldol反应和Mitsunobu型环脱水。所获得化合物的光谱数据和比旋光度与报道的天然存在的astakolactin的光谱数据和比旋光度非常匹配。











































 京公网安备 11010802027423号
京公网安备 11010802027423号