当前位置:
X-MOL 学术
›
Mol. Biosyst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Giardia intestinalis enolase and human plasminogen interaction
Molecular BioSystems Pub Date : 2017-07-17 00:00:00 , DOI: 10.1039/c7mb00252a R. Aguayo-Ortiz 1, 2, 3, 4, 5 , P. Meza-Cervantez 4, 6, 7, 8, 9 , R. Castillo 1, 3, 4, 5, 10 , A. Hernández-Campos 1, 3, 4, 5, 10 , L. Dominguez 1, 2, 3, 4, 5 , L. Yépez-Mulia 4, 6, 7, 8, 9
Molecular BioSystems Pub Date : 2017-07-17 00:00:00 , DOI: 10.1039/c7mb00252a R. Aguayo-Ortiz 1, 2, 3, 4, 5 , P. Meza-Cervantez 4, 6, 7, 8, 9 , R. Castillo 1, 3, 4, 5, 10 , A. Hernández-Campos 1, 3, 4, 5, 10 , L. Dominguez 1, 2, 3, 4, 5 , L. Yépez-Mulia 4, 6, 7, 8, 9
Affiliation
|
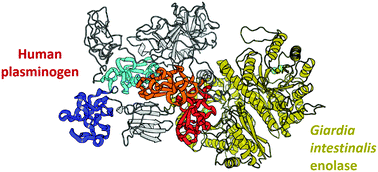
Giardia intestinalis is an intestinal parasite that causes diarrhea in humans and animals worldwide. The enolase of G. intestinalis (GiENO) participates in its glycolysis pathway and is abundantly expressed in the parasite cytosol; however, its localization on the surface of trophozoites and cysts has been demonstrated. Enolases from bacteria and parasites can have different functions and are considered moonlighting proteins, for example, as a cell surface plasminogen receptor. In relation to GiENO, no studies have been performed about its possible participation as a plasminogen receptor. In this work, we employed molecular docking and multiscale molecular dynamics (MD) simulations to explore the possible interactions of human plasminogen (HsPLG) with the open and closed GiENO conformations. Our proposed GiENO plasminogen binding site (PLGBs) was identified at Lys266 based on the sequence comparison with bacterial enolase known to act as a plasminogen receptor. Our docking results performed with multiple MD snapshots of the closed GiENO conformation showed that Lys266 preferentially binds to the K5 domain of HsPLG. On the other hand, open GiENO conformations from all-atom and coarse-grained simulations indicated a high preference of the HsPLG K4 domain for lysine residues 186 and 188. Furthermore, we identified a potential N-glycosylation site of GiENO which suggests a possible explanation for the parasite cell surface localization or host mucin oligosaccharide adhesion mechanism. Our study constitutes the first multiscale computational study to explore the plasminogen receptor function of GiENO for its further consideration as a potential therapeutic target for giardiasis treatment.
中文翻译:

贾第鞭毛虫肠烯醇酶和人类纤溶酶原相互作用的见解
贾第鞭毛虫肠道寄生虫是引起全世界人类和动物腹泻的一种肠道寄生虫。的烯醇化酶G.肠在其糖酵解途径(GiENO)参与和寄生虫细胞溶质大量表达; 然而,已证明其定位于滋养体和囊肿的表面。来自细菌和寄生虫的Enolases可能具有不同的功能,并且被认为是月光下的蛋白质,例如,作为细胞表面纤溶酶原受体。关于GiENO,尚未进行关于其作为纤溶酶原受体的可能参与的研究。在这项工作中,我们采用了分子对接和多尺度分子动力学(MD)模拟来探索人类纤溶酶原(HsPLG)与开放和封闭的GiENO构象。基于与已知充当纤溶酶原受体的细菌烯醇酶的序列比较,我们在Lys266上鉴定了我们提出的GiENO纤溶酶原结合位点(PLGB)。我们用封闭的GiENO构象的多个MD快照进行的对接结果表明,Lys266优先结合HsPLG的K5域。另一方面,打开来自全原子和粗粒度模拟的GiENO构象表明,HsPLG K4结构域对赖氨酸残基186和188的高度偏爱。此外,我们确定了GiENO的潜在N-糖基化位点,这为寄生虫细胞表面定位提供了可能的解释或宿主黏蛋白寡糖的黏附机制。我们的研究构成了第一个多尺度计算研究,旨在探索GiENO的纤溶酶原受体功能,以进一步考虑将其作为贾第鞭毛虫病治疗的潜在治疗靶标。
更新日期:2017-08-03
中文翻译:

贾第鞭毛虫肠烯醇酶和人类纤溶酶原相互作用的见解
贾第鞭毛虫肠道寄生虫是引起全世界人类和动物腹泻的一种肠道寄生虫。的烯醇化酶G.肠在其糖酵解途径(GiENO)参与和寄生虫细胞溶质大量表达; 然而,已证明其定位于滋养体和囊肿的表面。来自细菌和寄生虫的Enolases可能具有不同的功能,并且被认为是月光下的蛋白质,例如,作为细胞表面纤溶酶原受体。关于GiENO,尚未进行关于其作为纤溶酶原受体的可能参与的研究。在这项工作中,我们采用了分子对接和多尺度分子动力学(MD)模拟来探索人类纤溶酶原(HsPLG)与开放和封闭的GiENO构象。基于与已知充当纤溶酶原受体的细菌烯醇酶的序列比较,我们在Lys266上鉴定了我们提出的GiENO纤溶酶原结合位点(PLGB)。我们用封闭的GiENO构象的多个MD快照进行的对接结果表明,Lys266优先结合HsPLG的K5域。另一方面,打开来自全原子和粗粒度模拟的GiENO构象表明,HsPLG K4结构域对赖氨酸残基186和188的高度偏爱。此外,我们确定了GiENO的潜在N-糖基化位点,这为寄生虫细胞表面定位提供了可能的解释或宿主黏蛋白寡糖的黏附机制。我们的研究构成了第一个多尺度计算研究,旨在探索GiENO的纤溶酶原受体功能,以进一步考虑将其作为贾第鞭毛虫病治疗的潜在治疗靶标。










































 京公网安备 11010802027423号
京公网安备 11010802027423号