当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting βCys93 in hemoglobin S with an antisickling agent possessing dual allosteric and antioxidant effects
Metallomics ( IF 2.9 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1039/c7mt00104e Tigist Kassa 1, 2, 3, 4, 5 , Michael Brad Strader 1, 2, 3, 4, 5 , Akito Nakagawa 6, 7, 8, 9, 10 , Warren M. Zapol 6, 7, 8, 9, 10 , Abdu I. Alayash 1, 2, 3, 4, 5
Metallomics ( IF 2.9 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1039/c7mt00104e Tigist Kassa 1, 2, 3, 4, 5 , Michael Brad Strader 1, 2, 3, 4, 5 , Akito Nakagawa 6, 7, 8, 9, 10 , Warren M. Zapol 6, 7, 8, 9, 10 , Abdu I. Alayash 1, 2, 3, 4, 5
Affiliation
|
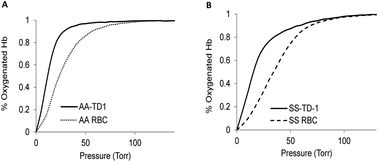
Sickle cell disease (SCD) is an inherited blood disorder caused by a β globin gene mutation of hemoglobin (HbS). The polymerization of deoxyHbS and its subsequent aggregation (into long fibers) is the primary molecular event which leads to red blood cell (RBC) sickling and ultimately hemolytic anemia. We have recently suggested that HbS oxidative toxicity may also contribute to SCD pathophysiology due to its defective pseudoperoxidase activity. As a consequence, a persistently higher oxidized ferryl heme is formed which irreversibly oxidizes “hotspot” residues (particularly βCys93) causing protein unfolding and subsequent heme loss. In this report we confirmed first, the allosteric effect of a newly developed reagent (di(5-(2,3-dihydro-1,4-benzodioxin-2-yl)-4H-1,2,4-triazol-3-yl)disulfide) (TD-1) on oxygen affinity within SS RBCs. There was a considerable left shift in oxygen equilibrium curves (OECs) representing treated SS cells. Under hypoxic conditions, TD-1 treatment of HbS resulted in an approximately 200 s increase in the delay time of HbS polymerization over the untreated HbS control. The effect of TD-1 binding to HbS was also tested on oxidative reactions by incrementally treating HbS with increasing hydrogen peroxide (H2O2) concentrations. Under these experimental conditions, ferryl levels were consistently reduced by approximately 35% in the presence of TD-1. Mass spectrometric analysis confirmed that upon binding to βCys93, TD-1 effectively blocked irreversible oxidation of this residue. In conclusion, TD-1 appears to shield βCys93 (the end point of radical formation in HbS) and when coupled with its allosteric effect on oxygen affinity may provide new therapeutic modalities for the treatment of SCD.
中文翻译:

用具有双重变构和抗氧化作用的抗镰刀蛋白靶向血红蛋白S中的βCys93
镰状细胞病(SCD)是由血红蛋白(HbS)的β珠蛋白基因突变引起的遗传性血液病。脱氧HbS的聚合及其随后的聚集(变成长纤维)是导致红细胞(RBC)镰刀化并最终导致溶血性贫血的主要分子事件。最近,我们建议HbS的氧化毒性也可能由于其伪过氧化物酶活性缺陷而导致SCD病理生理。结果,形成了持续较高的氧化的亚铁血红素,其不可逆地氧化了“热点”残基(尤其是βCys93),导致蛋白质解折叠和随后的血红素损失。在本报告中,我们首先确认了新开发的试剂(di(5-(2,3-dihydro-1,4-benzodioxin-2-yl)-4 H-1,2,4-三唑-3-基)二硫键(TD-1)对SS RBC中的氧亲和力。代表处理过的SS细胞的氧平衡曲线(OEC)有相当大的左移。在缺氧条件下,HbS的TD-1处理导致HbS聚合的延迟时间比未处理的HbS对照增加约200 s。还通过用增加的过氧化氢(H 2 O 2)浓度。在这些实验条件下,在存在TD-1的情况下,三聚氰胺水平持续降低约35%。质谱分析证实,与βCys93结合后,TD-1有效地阻止了该残基的不可逆氧化。总之,TD-1似乎可以屏蔽βCys93(HbS中自由基形成的终点),并且与它对氧亲和力的变构作用相结合,可能为SCD的治疗提供新的治疗方法。
更新日期:2017-08-03
中文翻译:

用具有双重变构和抗氧化作用的抗镰刀蛋白靶向血红蛋白S中的βCys93
镰状细胞病(SCD)是由血红蛋白(HbS)的β珠蛋白基因突变引起的遗传性血液病。脱氧HbS的聚合及其随后的聚集(变成长纤维)是导致红细胞(RBC)镰刀化并最终导致溶血性贫血的主要分子事件。最近,我们建议HbS的氧化毒性也可能由于其伪过氧化物酶活性缺陷而导致SCD病理生理。结果,形成了持续较高的氧化的亚铁血红素,其不可逆地氧化了“热点”残基(尤其是βCys93),导致蛋白质解折叠和随后的血红素损失。在本报告中,我们首先确认了新开发的试剂(di(5-(2,3-dihydro-1,4-benzodioxin-2-yl)-4 H-1,2,4-三唑-3-基)二硫键(TD-1)对SS RBC中的氧亲和力。代表处理过的SS细胞的氧平衡曲线(OEC)有相当大的左移。在缺氧条件下,HbS的TD-1处理导致HbS聚合的延迟时间比未处理的HbS对照增加约200 s。还通过用增加的过氧化氢(H 2 O 2)浓度。在这些实验条件下,在存在TD-1的情况下,三聚氰胺水平持续降低约35%。质谱分析证实,与βCys93结合后,TD-1有效地阻止了该残基的不可逆氧化。总之,TD-1似乎可以屏蔽βCys93(HbS中自由基形成的终点),并且与它对氧亲和力的变构作用相结合,可能为SCD的治疗提供新的治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号