当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted Delivery to Tumor-associated Pericytes via an Affibody with High Affinity for PDGFRβ Enhances the in vivo Antitumor Effects of Human TRAIL
Theranostics ( IF 12.4 ) Pub Date : 2017-06-01 , DOI: 10.7150/thno.19091 Ze Tao 1, 2 , Hao Yang 1 , Qiuxiao Shi 1, 2 , Qing Fan 1, 2 , Lin Wan 1 , Xiaofeng Lu 1
Theranostics ( IF 12.4 ) Pub Date : 2017-06-01 , DOI: 10.7150/thno.19091 Ze Tao 1, 2 , Hao Yang 1 , Qiuxiao Shi 1, 2 , Qing Fan 1, 2 , Lin Wan 1 , Xiaofeng Lu 1
Affiliation
|
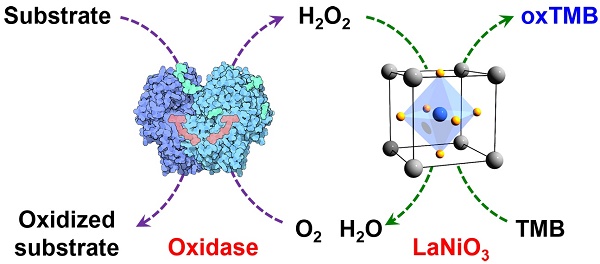
Human tumor necrosis factor-related apoptosis-inducing ligand (hTRAIL) has exhibited superior in vitro cytotoxicity in a variety of tumor cells. However, hTRAIL showed a disappointing anticancer effect in clinical trials, although hTRAIL-based regimens were well tolerated. One important reason might be that hTRAIL was largely trapped by its decoy receptors, which are ubiquitously expressed on normal cells. Tumor-targeted delivery might improve the tumor uptake and thus enhance the antitumor effect of hTRAIL. Platelet-derived growth factor receptor β (PDGFRβ)-expressing pericytes are enriched in tumor tissues derived both from patients with colon cancer and from mice bearing colorectal tumor xenografts. A ZPDGFRβ affibody showed high affinity (nM) for PDGFRβ and was predominantly distributed on tumor-associated PDGFRβ-positive pericytes. Co-administration with the ZPDGFRβ affibody did not significantly enhance the antitumor effect of hTRAIL in mice bearing tumor xenografts. Fusion to the ZPDGFRβ affibody endows hTRAIL with PDGFRβ-binding ability but does not interfere with its death receptor binding and activation. The fused ZPDGFRβ affibody mediated PDGFRβ-dependent binding of hTRAIL to pericytes. In addition, hTRAIL bound on pericytes could kill tumor cells through juxtatropic activity or exhibit cytotoxicity in tumor cells after being released from pericytes. Intravenously injected hTRAIL fused to ZPDGFRβ affibody initially accumulated on tumor-associated pericytes and then diffused to the tumor parenchyma over time. Fusion to the ZPDGFRβ affibody increased the tumor uptake of hTRAIL, thus enhancing the antitumor effect of hTRAIL in mice bearing tumor xenografts. These results demonstrate that pericyte-targeted delivery mediated by a ZPDGFRβ affibody is an alternative strategy for tumor-targeted delivery of anticancer agents.
中文翻译:

通过对 PDGFRβ 高亲和力的亲和体靶向递送至肿瘤相关周细胞增强人 TRAIL 的体内抗肿瘤作用
人肿瘤坏死因子相关凋亡诱导配体(hTRAIL)在多种肿瘤细胞中表现出优异的体外细胞毒性。然而,尽管基于 hTRAIL 的治疗方案具有良好的耐受性,但 hTRAIL 在临床试验中显示出令人失望的抗癌效果。一个重要的原因可能是 hTRAIL 很大程度上被其诱饵受体所捕获,而这些受体在正常细胞上普遍表达。肿瘤靶向递送可能会改善肿瘤的摄取,从而增强 hTRAIL 的抗肿瘤作用。表达血小板源性生长因子受体β(PDGFRβ)的周细胞在来自结肠癌患者和带有结直肠肿瘤异种移植物的小鼠的肿瘤组织中富集。 AZ PDGFRβ亲和体对PDGFRβ表现出高亲和力(nM),并且主要分布在肿瘤相关PDGFRβ阳性周细胞上。与 Z PDGFRβ亲和体共同给药并没有显着增强 hTRAIL 在携带肿瘤异种移植物的小鼠中的抗肿瘤作用。与 Z PDGFRβ亲和体的融合赋予 hTRAIL 与 PDGFRβ 的结合能力,但不会干扰其死亡受体的结合和激活。融合的 Z PDGFRβ亲和体介导 hTRAIL 与周细胞的 PDGFRβ 依赖性结合。此外,结合在周细胞上的hTRAIL可以通过并列活性杀死肿瘤细胞,或者从周细胞释放后在肿瘤细胞中表现出细胞毒性。静脉注射与 Z PDGFRβ亲和体融合的 hTRAIL 最初积聚在肿瘤相关周细胞上,然后随着时间的推移扩散到肿瘤实质。 与 Z PDGFRβ亲和体的融合增加了肿瘤对 hTRAIL 的摄取,从而增强了 hTRAIL 在携带肿瘤异种移植物的小鼠中的抗肿瘤作用。这些结果表明,由 Z PDGFRβ亲和体介导的周细胞靶向递送是肿瘤靶向递送抗癌药物的替代策略。
更新日期:2017-08-02
中文翻译:

通过对 PDGFRβ 高亲和力的亲和体靶向递送至肿瘤相关周细胞增强人 TRAIL 的体内抗肿瘤作用
人肿瘤坏死因子相关凋亡诱导配体(hTRAIL)在多种肿瘤细胞中表现出优异的体外细胞毒性。然而,尽管基于 hTRAIL 的治疗方案具有良好的耐受性,但 hTRAIL 在临床试验中显示出令人失望的抗癌效果。一个重要的原因可能是 hTRAIL 很大程度上被其诱饵受体所捕获,而这些受体在正常细胞上普遍表达。肿瘤靶向递送可能会改善肿瘤的摄取,从而增强 hTRAIL 的抗肿瘤作用。表达血小板源性生长因子受体β(PDGFRβ)的周细胞在来自结肠癌患者和带有结直肠肿瘤异种移植物的小鼠的肿瘤组织中富集。 AZ PDGFRβ亲和体对PDGFRβ表现出高亲和力(nM),并且主要分布在肿瘤相关PDGFRβ阳性周细胞上。与 Z PDGFRβ亲和体共同给药并没有显着增强 hTRAIL 在携带肿瘤异种移植物的小鼠中的抗肿瘤作用。与 Z PDGFRβ亲和体的融合赋予 hTRAIL 与 PDGFRβ 的结合能力,但不会干扰其死亡受体的结合和激活。融合的 Z PDGFRβ亲和体介导 hTRAIL 与周细胞的 PDGFRβ 依赖性结合。此外,结合在周细胞上的hTRAIL可以通过并列活性杀死肿瘤细胞,或者从周细胞释放后在肿瘤细胞中表现出细胞毒性。静脉注射与 Z PDGFRβ亲和体融合的 hTRAIL 最初积聚在肿瘤相关周细胞上,然后随着时间的推移扩散到肿瘤实质。 与 Z PDGFRβ亲和体的融合增加了肿瘤对 hTRAIL 的摄取,从而增强了 hTRAIL 在携带肿瘤异种移植物的小鼠中的抗肿瘤作用。这些结果表明,由 Z PDGFRβ亲和体介导的周细胞靶向递送是肿瘤靶向递送抗癌药物的替代策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号