当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MAO inhibitory activity of bromo-2-phenylbenzofurans: synthesis, in vitro study, and docking calculations
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-07-07 00:00:00 , DOI: 10.1039/c7md00311k G. L. Delogu 1, 2, 3 , F. Pintus 1, 2, 3 , L. Mayán 4, 5, 6, 7, 8 , M. J. Matos 5, 6, 8, 9, 10 , S. Vilar 5, 6, 8, 9, 10 , J. Munín 4, 5, 6, 8, 9 , J. A. Fontenla 5, 6, 7, 8, 10 , G. Hripcsak 11, 12, 13, 14, 15 , F. Borges 16, 17, 18 , D. Viña 4, 5, 6
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-07-07 00:00:00 , DOI: 10.1039/c7md00311k G. L. Delogu 1, 2, 3 , F. Pintus 1, 2, 3 , L. Mayán 4, 5, 6, 7, 8 , M. J. Matos 5, 6, 8, 9, 10 , S. Vilar 5, 6, 8, 9, 10 , J. Munín 4, 5, 6, 8, 9 , J. A. Fontenla 5, 6, 7, 8, 10 , G. Hripcsak 11, 12, 13, 14, 15 , F. Borges 16, 17, 18 , D. Viña 4, 5, 6
Affiliation
|
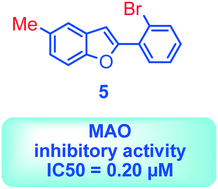
Monoamine oxidase (MAO) is an enzyme responsible for metabolism of monoamine neurotransmitters which play an important role in brain development and function. This enzyme exists in two isoforms, and it has been demonstrated that MAO-B activity, but not MAO-A activity, increases with aging. MAO inhibitors show clinical value because besides the monoamine level regulation they reduce the formation of by-products of the MAO catalytic cycle, which are toxic to the brain. A series of 2-phenylbenzofuran derivatives was designed, synthesized and evaluated against hMAO-A and hMAO-B enzymes. A bromine substituent was introduced in the 2-phenyl ring, whereas position 5 or 7 of the benzofuran moiety was substituted with a methyl group. Most of the tested compounds inhibited preferentially MAO-B in a reversible manner, with IC50 values in the low micro or nanomolar range. The 2-(2′-bromophenyl)-5-methylbenzofuran (5) was the most active compound identified (IC50 = 0.20 μM). In addition, none of the studied compounds showed cytotoxic activity against the human neuroblastoma cell line SH-SY5Y. Molecular docking simulations were used to explain the observed hMAO-B structure–activity relationship for this type of compounds.
中文翻译:

溴-2-苯基苯并呋喃的MAO抑制活性:合成,体外研究和对接计算
单胺氧化酶(MAO)是负责单胺神经递质代谢的酶,其在大脑发育和功能中起重要作用。该酶以两种同工型存在,并且已经证明,MAO-B活性会随着年龄的增长而增加,而不是MAO-A活性。MAO抑制剂具有临床价值,因为除单胺水平调节外,它们还可以减少MAO催化循环副产物的形成,这些副产物对大脑有毒。设计,合成了一系列2-苯基苯并呋喃衍生物,并针对hMAO-A和hMAO-B酶进行了评估。在2-苯环中引入了溴取代基,而苯并呋喃部分的5或7位被甲基取代。大多数测试化合物以可逆的方式优先抑制MAO-B,IC 50为数值在低微摩尔或纳摩尔范围内。2-(2'-溴苯基)-5-甲基苯并呋喃(5)是鉴定出的最具活性的化合物(IC 50 = 0.20μM)。另外,所研究的化合物均未显示出对人神经母细胞瘤细胞系SH-SY5Y的细胞毒活性。分子对接模拟用于解释这种化合物观察到的hMAO-B结构与活性之间的关系。
更新日期:2017-08-02
中文翻译:

溴-2-苯基苯并呋喃的MAO抑制活性:合成,体外研究和对接计算
单胺氧化酶(MAO)是负责单胺神经递质代谢的酶,其在大脑发育和功能中起重要作用。该酶以两种同工型存在,并且已经证明,MAO-B活性会随着年龄的增长而增加,而不是MAO-A活性。MAO抑制剂具有临床价值,因为除单胺水平调节外,它们还可以减少MAO催化循环副产物的形成,这些副产物对大脑有毒。设计,合成了一系列2-苯基苯并呋喃衍生物,并针对hMAO-A和hMAO-B酶进行了评估。在2-苯环中引入了溴取代基,而苯并呋喃部分的5或7位被甲基取代。大多数测试化合物以可逆的方式优先抑制MAO-B,IC 50为数值在低微摩尔或纳摩尔范围内。2-(2'-溴苯基)-5-甲基苯并呋喃(5)是鉴定出的最具活性的化合物(IC 50 = 0.20μM)。另外,所研究的化合物均未显示出对人神经母细胞瘤细胞系SH-SY5Y的细胞毒活性。分子对接模拟用于解释这种化合物观察到的hMAO-B结构与活性之间的关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号