当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Versatile Site-Selective Protein Reaction Guided by WW Domain–Peptide Motif Interaction
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-07-29 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00334 Miao Liu 1 , Zeyang Ji 2 , Mingjie Zhang 2 , Jiang Xia 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-07-29 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00334 Miao Liu 1 , Zeyang Ji 2 , Mingjie Zhang 2 , Jiang Xia 1
Affiliation
|
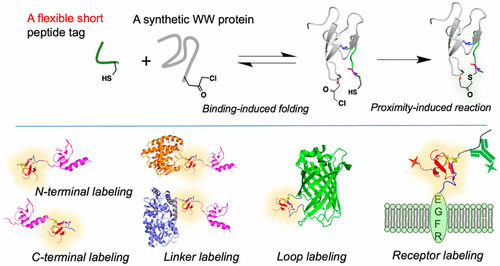
A short, flexible, and unstructured peptide tag that has versatile and facile use in protein labeling applications is highly desirable. Here, we report an 11-residue peptide tag with an internal cysteine (a W-tag, derived from a Comm PY peptide motif that is known to bind with Nedd4 WW3* domain) that can be installed at different regions of the target protein without compromising its covalent reactivity with the reactive label (a 35-residue synthetic Nedd4 WW3* domain derivative). This versatility is explained by the unique structural features of the reaction. NMR analysis reveals that both the W-tag peptide and reactive Nedd4 WW3* protein are unstructured before they encounter each other. The binding interaction of the two induces noticeable structural changes and promotes global folding. Consequently, the reactive cysteine residue at W-tag and the electrophilic chloroacetyl group at Nedd4 WW3* domain are positioned to be in close proximity, inducing an intermolecular covalent cross-linking. The covalent linkage in turn stabilizes the folding of the protein complex. This unique multistep mechanism renders this labeling reaction amenable to different sites of the proteins of interest: installation of the tag at N- and C-termini, in the flexible linker region, in the loop region, and the extracellular terminus of target proteins exhibited comparable reactivity. This work therefore represents the first proximity-induced cysteine reaction based on the unique binding features of WW domains that demonstrates unprecedented versatility.
中文翻译:

WW域–肽基序相互作用指导的多功能位点选择蛋白反应
在蛋白质标记应用中具有通用且简便的用途的短的,柔性的和非结构化的肽标签是非常需要的。在此,我们报告了一个11残基的肽标签,该标签具有内部半胱氨酸(W标签,源自Comm PY肽基序,已知与Nedd4 WW3 *域结合),可以安装在目标蛋白的不同区域,而无需损害其与反应性标记(一种35残基的合成Nedd4 WW3 *域衍生物)的共价反应性。反应的独特结构特征解释了这种多功能性。NMR分析显示,W标签肽和反应性Nedd4 WW3 *蛋白在彼此相遇之前都是无结构的。两者的结合相互作用引起明显的结构变化并促进整体折叠。所以,W标签上的反应性半胱氨酸残基和Nedd4 WW3 *域上的亲电子氯乙酰基位置紧密接近,从而诱导了分子间共价交联。共价键进而稳定了蛋白质复合物的折叠。这种独特的多步机制使该标记反应适合目标蛋白质的不同位点:将标签安装在N-和C-末端,柔性接头区域,环区域中,并且靶蛋白质的细胞外末端具有可比性反应性。因此,这项工作代表了基于WW域独特的结合特征的第一个邻近诱导的半胱氨酸反应,该反应具有前所未有的多功能性。诱导分子间共价交联。共价键进而稳定了蛋白质复合物的折叠。这种独特的多步机制使该标记反应适合目标蛋白质的不同位点:将标签安装在N-和C-末端,柔性接头区域,环区域中,并且靶蛋白质的细胞外末端具有可比性反应性。因此,这项工作代表了基于WW域独特的结合特征的第一个邻近诱导的半胱氨酸反应,该反应具有前所未有的多功能性。诱导分子间共价交联。共价键进而稳定了蛋白质复合物的折叠。这种独特的多步机制使该标记反应适合目标蛋白质的不同位点:将标签安装在N-和C-末端,柔性接头区域,环区域中,并且靶蛋白质的细胞外末端具有可比性反应性。因此,这项工作代表了基于WW域独特的结合特征的第一个邻近诱导的半胱氨酸反应,该反应具有前所未有的多功能性。在柔性接头区域,环区域和N-和C-末端的标签安装以及靶蛋白的细胞外末端均表现出可比的反应性。因此,这项工作代表了基于WW域独特的结合特征的第一个邻近诱导的半胱氨酸反应,展现出前所未有的多功能性。在柔性接头区域,环区域和N-和C-末端的标签安装以及靶蛋白的细胞外末端均表现出可比的反应性。因此,这项工作代表了基于WW域独特的结合特征的第一个邻近诱导的半胱氨酸反应,该反应具有前所未有的多功能性。
更新日期:2017-07-30
中文翻译:

WW域–肽基序相互作用指导的多功能位点选择蛋白反应
在蛋白质标记应用中具有通用且简便的用途的短的,柔性的和非结构化的肽标签是非常需要的。在此,我们报告了一个11残基的肽标签,该标签具有内部半胱氨酸(W标签,源自Comm PY肽基序,已知与Nedd4 WW3 *域结合),可以安装在目标蛋白的不同区域,而无需损害其与反应性标记(一种35残基的合成Nedd4 WW3 *域衍生物)的共价反应性。反应的独特结构特征解释了这种多功能性。NMR分析显示,W标签肽和反应性Nedd4 WW3 *蛋白在彼此相遇之前都是无结构的。两者的结合相互作用引起明显的结构变化并促进整体折叠。所以,W标签上的反应性半胱氨酸残基和Nedd4 WW3 *域上的亲电子氯乙酰基位置紧密接近,从而诱导了分子间共价交联。共价键进而稳定了蛋白质复合物的折叠。这种独特的多步机制使该标记反应适合目标蛋白质的不同位点:将标签安装在N-和C-末端,柔性接头区域,环区域中,并且靶蛋白质的细胞外末端具有可比性反应性。因此,这项工作代表了基于WW域独特的结合特征的第一个邻近诱导的半胱氨酸反应,该反应具有前所未有的多功能性。诱导分子间共价交联。共价键进而稳定了蛋白质复合物的折叠。这种独特的多步机制使该标记反应适合目标蛋白质的不同位点:将标签安装在N-和C-末端,柔性接头区域,环区域中,并且靶蛋白质的细胞外末端具有可比性反应性。因此,这项工作代表了基于WW域独特的结合特征的第一个邻近诱导的半胱氨酸反应,该反应具有前所未有的多功能性。诱导分子间共价交联。共价键进而稳定了蛋白质复合物的折叠。这种独特的多步机制使该标记反应适合目标蛋白质的不同位点:将标签安装在N-和C-末端,柔性接头区域,环区域中,并且靶蛋白质的细胞外末端具有可比性反应性。因此,这项工作代表了基于WW域独特的结合特征的第一个邻近诱导的半胱氨酸反应,该反应具有前所未有的多功能性。在柔性接头区域,环区域和N-和C-末端的标签安装以及靶蛋白的细胞外末端均表现出可比的反应性。因此,这项工作代表了基于WW域独特的结合特征的第一个邻近诱导的半胱氨酸反应,展现出前所未有的多功能性。在柔性接头区域,环区域和N-和C-末端的标签安装以及靶蛋白的细胞外末端均表现出可比的反应性。因此,这项工作代表了基于WW域独特的结合特征的第一个邻近诱导的半胱氨酸反应,该反应具有前所未有的多功能性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号