当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Methylene Capping: A General Strategy for Efficient Stereoretentive Catalytic Olefin Metathesis. The Concept, Methodological Implications, and Applications to Synthesis of Biologically Active Compounds
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-27 , DOI: 10.1021/jacs.7b06552 Chaofan Xu 1 , Xiao Shen 1 , Amir H. Hoveyda 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-27 , DOI: 10.1021/jacs.7b06552 Chaofan Xu 1 , Xiao Shen 1 , Amir H. Hoveyda 1
Affiliation
|
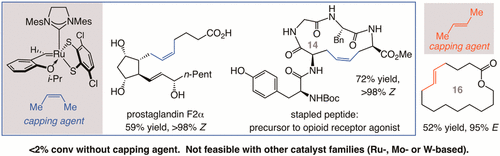
In situ methylene capping is introduced as a practical and broadly applicable strategy that can expand the scope of catalyst-controlled stereoselective olefin metathesis considerably. By incorporation of commercially available Z-butene together with robust and readily accessible Ru-based dithiolate catalysts developed in these laboratories, a large variety of transformations can be made to proceed with terminal alkenes, without the need for a priori synthesis of a stereochemically defined disubstituted olefin. Reactions thus proceed with significantly higher efficiency and Z selectivity as compared to when other Ru-, Mo-, or W-based complexes are utilized. Cross-metathesis with olefins that contain a carboxylic acid, an aldehyde, an allylic alcohol, an aryl olefin, an α substituent, or amino acid residues was carried out to generate the desired products in 47-88% yield and 90:10 to >98:2 Z:E selectivity. Transformations were equally efficient and stereoselective with a ∼70:30 Z-:E-butene mixture, which is a byproduct of crude oil cracking. The in situ methylene capping strategy was used with the same Ru catechothiolate complex (no catalyst modification necessary) to perform ring-closing metathesis reactions, generating 14- to 21-membered ring macrocyclic alkenes in 40-70% yield and 96:4-98:2 Z:E selectivity; here too, reactions were more efficient and Z-selective than when the other catalyst classes are employed. The utility of the approach is highlighted by applications to efficient and stereoselective syntheses of several biologically active molecules. This includes a platelet aggregate inhibitor and two members of the prostaglandin family of compounds by catalytic cross-metathesis reactions, and a strained 14-membered ring stapled peptide by means of macrocyclic ring-closing metathesis. The approach presented herein is likely to have a notable effect on broadening the scope of olefin metathesis, as the stability of methylidene complexes is a generally debilitating issue with all types of catalyst systems. Illustrative examples of kinetically controlled E-selective cross-metathesis and macrocyclic ring-closing reactions, where E-butene serves as the methylene capping agent, are provided.
中文翻译:

原位亚甲基封端:高效立体保持催化烯烃复分解的一般策略。生物活性化合物合成的概念、方法学意义和应用
原位亚甲基封端作为一种实用且广泛适用的策略被引入,可以显着扩大催化剂控制的立体选择性烯烃复分解的范围。通过将市售的 Z-丁烯与这些实验室开发的坚固且易于获得的基于 Ru 的二硫醇催化剂结合,可以进行多种转化以处理末端烯烃,而无需先验合成立体化学定义的双取代烯烃。因此,与使用其他基于 Ru、Mo 或 W 的配合物相比,反应以显着更高的效率和 Z 选择性进行。与含有羧酸、醛、烯丙醇、芳基烯烃、α取代基的烯烃交叉复分解,或氨基酸残基以 47-88% 的产率和 90:10 至 >98:2 的 Z:E 选择性生成所需产物。使用 70:30 Z-:E-丁烯混合物(原油裂解的副产品),转化同样有效且具有立体选择性。原位亚甲基封端策略与相同的儿茶酚硫醇配合物(无需催化剂修饰)一起进行闭环复分解反应,以 40-70% 的产率和 96:4-98 的产率生成 14 至 21 元环大环烯烃:2 Z:E 选择性;与使用其他催化剂类别时相比,这里的反应也更有效且具有 Z 选择性。该方法的实用性通过应用于几种生物活性分子的有效和立体选择性合成而突出。这包括通过催化交叉复分解反应产生的血小板聚集抑制剂和前列腺素家族化合物的两个成员,以及通过大环闭环复分解产生的紧张的 14 元环钉合肽。本文提出的方法可能对拓宽烯烃复分解的范围产生显着影响,因为亚甲基配合物的稳定性通常是所有类型催化剂体系的弱点。提供了动力学控制的 E-选择性交叉复分解和大环闭环反应的说明性例子,其中 E-丁烯用作亚甲基封端剂。本文提出的方法可能对拓宽烯烃复分解的范围产生显着影响,因为亚甲基配合物的稳定性通常是所有类型催化剂体系的弱点。提供了动力学控制的 E-选择性交叉复分解和大环闭环反应的说明性例子,其中 E-丁烯用作亚甲基封端剂。本文提出的方法可能对拓宽烯烃复分解的范围产生显着影响,因为亚甲基配合物的稳定性通常是所有类型催化剂体系的弱点。提供了动力学控制的 E-选择性交叉复分解和大环闭环反应的说明性例子,其中 E-丁烯用作亚甲基封端剂。
更新日期:2017-07-27
中文翻译:

原位亚甲基封端:高效立体保持催化烯烃复分解的一般策略。生物活性化合物合成的概念、方法学意义和应用
原位亚甲基封端作为一种实用且广泛适用的策略被引入,可以显着扩大催化剂控制的立体选择性烯烃复分解的范围。通过将市售的 Z-丁烯与这些实验室开发的坚固且易于获得的基于 Ru 的二硫醇催化剂结合,可以进行多种转化以处理末端烯烃,而无需先验合成立体化学定义的双取代烯烃。因此,与使用其他基于 Ru、Mo 或 W 的配合物相比,反应以显着更高的效率和 Z 选择性进行。与含有羧酸、醛、烯丙醇、芳基烯烃、α取代基的烯烃交叉复分解,或氨基酸残基以 47-88% 的产率和 90:10 至 >98:2 的 Z:E 选择性生成所需产物。使用 70:30 Z-:E-丁烯混合物(原油裂解的副产品),转化同样有效且具有立体选择性。原位亚甲基封端策略与相同的儿茶酚硫醇配合物(无需催化剂修饰)一起进行闭环复分解反应,以 40-70% 的产率和 96:4-98 的产率生成 14 至 21 元环大环烯烃:2 Z:E 选择性;与使用其他催化剂类别时相比,这里的反应也更有效且具有 Z 选择性。该方法的实用性通过应用于几种生物活性分子的有效和立体选择性合成而突出。这包括通过催化交叉复分解反应产生的血小板聚集抑制剂和前列腺素家族化合物的两个成员,以及通过大环闭环复分解产生的紧张的 14 元环钉合肽。本文提出的方法可能对拓宽烯烃复分解的范围产生显着影响,因为亚甲基配合物的稳定性通常是所有类型催化剂体系的弱点。提供了动力学控制的 E-选择性交叉复分解和大环闭环反应的说明性例子,其中 E-丁烯用作亚甲基封端剂。本文提出的方法可能对拓宽烯烃复分解的范围产生显着影响,因为亚甲基配合物的稳定性通常是所有类型催化剂体系的弱点。提供了动力学控制的 E-选择性交叉复分解和大环闭环反应的说明性例子,其中 E-丁烯用作亚甲基封端剂。本文提出的方法可能对拓宽烯烃复分解的范围产生显着影响,因为亚甲基配合物的稳定性通常是所有类型催化剂体系的弱点。提供了动力学控制的 E-选择性交叉复分解和大环闭环反应的说明性例子,其中 E-丁烯用作亚甲基封端剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号