当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Retuning the Catalytic Bias and Overpotential of a [NiFe]-Hydrogenase via a Single Amino Acid Exchange at the Electron Entry/Exit Site
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1021/jacs.7b03611 Hope Adamson 1 , Martin Robinson 2 , John J. Wright 3 , Lindsey A. Flanagan 1 , Julia Walton 1 , Darrell Elton 4 , David J. Gavaghan 2 , Alan M. Bond 5 , Maxie M. Roessler 3 , Alison Parkin 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1021/jacs.7b03611 Hope Adamson 1 , Martin Robinson 2 , John J. Wright 3 , Lindsey A. Flanagan 1 , Julia Walton 1 , Darrell Elton 4 , David J. Gavaghan 2 , Alan M. Bond 5 , Maxie M. Roessler 3 , Alison Parkin 1
Affiliation
|
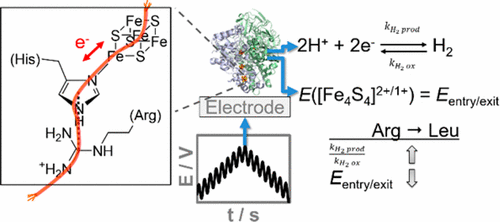
The redox chemistry of the electron entry/exit site in Escherichia coli hydrogenase-1 is shown to play a vital role in tuning biocatalysis. Inspired by nature, we generate a HyaA-R193L variant to disrupt a proposed Arg–His cation−π interaction in the secondary coordination sphere of the outermost, “distal”, iron–sulfur cluster. This rewires the enzyme, enhancing the relative rate of H2 production and the thermodynamic efficiency of H2 oxidation catalysis. On the basis of Fourier transformed alternating current voltammetry measurements, we relate these changes in catalysis to a shift in the distal [Fe4S4]2+/1+ redox potential, a previously experimentally inaccessible parameter. Thus, metalloenzyme chemistry is shown to be tuned by the second coordination sphere of an electron transfer site distant from the catalytic center.
中文翻译:

通过电子进/出位处的单个氨基酸交换,重新调节[NiFe]-氢化酶的催化偏差和超电势
已显示大肠杆菌氢化酶-1中电子进入/退出位点的氧化还原化学在调节生物催化中起着至关重要的作用。受自然界的启发,我们产生了一个HyaA-R193L变体,以破坏最外侧的“远端”铁-硫簇的二级配位域中拟议的Arg-His阳离子-π相互作用。这使酶重新连接,从而提高了H 2产生的相对速率和H 2氧化催化的热力学效率。在傅立叶变换交流伏安法测量的基础上,我们将催化的这些变化与远端[Fe 4 S 4 ] 2 + / 1 +的移动相关联氧化还原电位,以前无法通过实验获得的参数。因此,金属酶化学显示出是通过远离催化中心的电子转移位点的第二配位球来调节的。
更新日期:2017-07-28
中文翻译:

通过电子进/出位处的单个氨基酸交换,重新调节[NiFe]-氢化酶的催化偏差和超电势
已显示大肠杆菌氢化酶-1中电子进入/退出位点的氧化还原化学在调节生物催化中起着至关重要的作用。受自然界的启发,我们产生了一个HyaA-R193L变体,以破坏最外侧的“远端”铁-硫簇的二级配位域中拟议的Arg-His阳离子-π相互作用。这使酶重新连接,从而提高了H 2产生的相对速率和H 2氧化催化的热力学效率。在傅立叶变换交流伏安法测量的基础上,我们将催化的这些变化与远端[Fe 4 S 4 ] 2 + / 1 +的移动相关联氧化还原电位,以前无法通过实验获得的参数。因此,金属酶化学显示出是通过远离催化中心的电子转移位点的第二配位球来调节的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号