当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
With unprotected amino acids to tetrazolo peptidomimetics
Chemical Communications ( IF 4.3 ) Pub Date : 2017-07-12 00:00:00 , DOI: 10.1039/c7cc03370b Rudrakshula Madhavachary 1, 2, 3, 4 , Qian Wang 1, 2, 3, 4 , Alexander Dömling 1, 2, 3, 4
Chemical Communications ( IF 4.3 ) Pub Date : 2017-07-12 00:00:00 , DOI: 10.1039/c7cc03370b Rudrakshula Madhavachary 1, 2, 3, 4 , Qian Wang 1, 2, 3, 4 , Alexander Dömling 1, 2, 3, 4
Affiliation
|
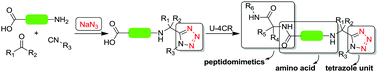
Here we describe the direct usage of C,N-unprotected amino acids in Ugi-tetrazole reactions to produce a novel class of acid-tetrazole compounds. Surprisingly, only the tetrazole Ugi product is found and not traces of other possible Ugi type reactions. Based on this reaction pathway we have designed the synthesis of novel tetrazole-peptidomimetics. A high level of structural diversity can be achieved using this isocyanide based multicomponent reaction (IMCR), providing a platform for the production of functionalized building blocks for novel bioactive molecules and nontraditional scaffolds which previously were not accessible.
中文翻译:

具有不受保护的氨基酸到四唑拟肽
在这里,我们描述了在Ugi-四唑反应中直接使用C,N-未保护的氨基酸来生产一类新型的酸-四唑化合物。令人惊讶地,仅发现四唑Ugi产物,而没有发现其他可能的Ugi型反应的痕迹。基于该反应途径,我们设计了新颖的四唑-拟肽类似物的合成。使用这种基于异氰化物的多组分反应(IMCR),可以实现高水平的结构多样性,为生产新型生物活性分子和以前无法获得的非传统支架的功能化构建基块提供了平台。
更新日期:2017-07-28
中文翻译:

具有不受保护的氨基酸到四唑拟肽
在这里,我们描述了在Ugi-四唑反应中直接使用C,N-未保护的氨基酸来生产一类新型的酸-四唑化合物。令人惊讶地,仅发现四唑Ugi产物,而没有发现其他可能的Ugi型反应的痕迹。基于该反应途径,我们设计了新颖的四唑-拟肽类似物的合成。使用这种基于异氰化物的多组分反应(IMCR),可以实现高水平的结构多样性,为生产新型生物活性分子和以前无法获得的非传统支架的功能化构建基块提供了平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号