当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single antibody–antigen interactions monitored via transient ionic current recording using nanopore sensors
Chemical Communications ( IF 4.9 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1039/c7cc03927a Yi-Lun Ying 1, 2, 3, 4, 5 , Ru-Jia Yu 1, 2, 3, 4, 5 , Yong-Xu Hu 1, 2, 3, 4, 5 , Rui Gao 1, 2, 3, 4, 5 , Yi-Tao Long 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1039/c7cc03927a Yi-Lun Ying 1, 2, 3, 4, 5 , Ru-Jia Yu 1, 2, 3, 4, 5 , Yong-Xu Hu 1, 2, 3, 4, 5 , Rui Gao 1, 2, 3, 4, 5 , Yi-Tao Long 1, 2, 3, 4, 5
Affiliation
|
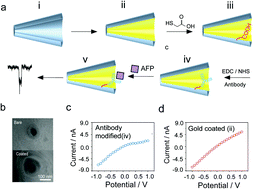
Understanding the single molecular protein–protein interaction has great significance in evaluating the affinity of a specific antibody. Herein, the interaction between single α-fetal protein (AFP) and its antibody was monitored via transient ionic current recording by using the antibody functionalized nanopore sensors. More importantly, the kinetic evaluation was performed at the single molecule level to determine the dissociation constant of this interaction. This method enables the monitoring of the kinetic antigen–antibody interaction in their heterogenetic state without any labelling. Our results provided new insights into the evaluation of the antibody's binding affinity and more into the development of immunoassays for diagnostics.
中文翻译:

通过使用纳米孔传感器的瞬时离子电流记录监控单个抗体-抗原的相互作用
了解单分子蛋白之间的相互作用对评估特定抗体的亲和力具有重要意义。在此,通过使用抗体功能化的纳米孔传感器,通过瞬时离子电流记录来监测单个α-胎儿蛋白(AFP)与其抗体之间的相互作用。更重要的是,在单分子水平上进行了动力学评估,以确定这种相互作用的解离常数。这种方法可以监测处于异质状态的动态抗原-抗体相互作用,而无需任何标记。我们的结果为评估抗体的结合亲和力提供了新的见识,并为诊断免疫分析的发展提供了新的见识。
更新日期:2017-07-28
中文翻译:

通过使用纳米孔传感器的瞬时离子电流记录监控单个抗体-抗原的相互作用
了解单分子蛋白之间的相互作用对评估特定抗体的亲和力具有重要意义。在此,通过使用抗体功能化的纳米孔传感器,通过瞬时离子电流记录来监测单个α-胎儿蛋白(AFP)与其抗体之间的相互作用。更重要的是,在单分子水平上进行了动力学评估,以确定这种相互作用的解离常数。这种方法可以监测处于异质状态的动态抗原-抗体相互作用,而无需任何标记。我们的结果为评估抗体的结合亲和力提供了新的见识,并为诊断免疫分析的发展提供了新的见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号