当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral phosphoric acid-catalyzed desymmetrizative glycosylation of 2-deoxystreptamine and its application to aminoglycoside synthesis
Chemical Communications ( IF 4.9 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1039/c7cc05052f Jeonghyo Lee 1, 2, 3, 4 , Alina Borovika 4, 5, 6 , Yaroslav Khomutnyk 1, 2, 3, 4 , Pavel Nagorny 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1039/c7cc05052f Jeonghyo Lee 1, 2, 3, 4 , Alina Borovika 4, 5, 6 , Yaroslav Khomutnyk 1, 2, 3, 4 , Pavel Nagorny 1, 2, 3, 4
Affiliation
|
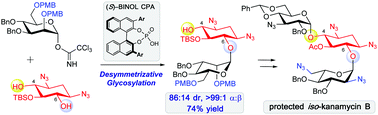
This work describes chiral phosphoric acid (CPA)-catalyzed desymmetrizative glycosylation of meso-diol derived from 2-deoxystreptamine. The chirality of CPA dictates the outcome of the glycosylation reactions, and the use of enantiomeric CPAs results in either C4-glycosylated (67 :
: 33 d.r.) or C6-glycosylated (86
33 d.r.) or C6-glycosylated (86 :
: 14 d.r.) 2-deoxystreptamines. These glycosylated products can be converted to aminoglycosides, and the application of this strategy to the synthesis of protected iso-neamine and iso-kanamycin B with inverted connection at the C4 and C6 positions is described.
14 d.r.) 2-deoxystreptamines. These glycosylated products can be converted to aminoglycosides, and the application of this strategy to the synthesis of protected iso-neamine and iso-kanamycin B with inverted connection at the C4 and C6 positions is described.
中文翻译:

手性磷酸催化2-脱氧链烷胺的不对称糖基化及其在氨基糖苷合成中的应用
这项工作描述了手性磷酸(CPA)催化衍生自2-脱氧链胺的内消旋二醇的不对称糖基化。CPA的手性决定了糖基化反应的结果,在上述任何一种C4-糖基化中使用的对映体会计师结果(67 :
: 33 DR)或C6-糖基化(86
33 DR)或C6-糖基化(86  :
: 14 DR)2- deoxystreptamines。这些糖基化的产物可以转化为氨基糖苷,并且描述了该策略在C4和C6位具有反向连接的保护的异亚胺和异卡那霉素B的合成中的应用。
14 DR)2- deoxystreptamines。这些糖基化的产物可以转化为氨基糖苷,并且描述了该策略在C4和C6位具有反向连接的保护的异亚胺和异卡那霉素B的合成中的应用。
更新日期:2017-07-28
 :
: 33 d.r.) or C6-glycosylated (86
33 d.r.) or C6-glycosylated (86 :
: 14 d.r.) 2-deoxystreptamines. These glycosylated products can be converted to aminoglycosides, and the application of this strategy to the synthesis of protected iso-neamine and iso-kanamycin B with inverted connection at the C4 and C6 positions is described.
14 d.r.) 2-deoxystreptamines. These glycosylated products can be converted to aminoglycosides, and the application of this strategy to the synthesis of protected iso-neamine and iso-kanamycin B with inverted connection at the C4 and C6 positions is described.
中文翻译:

手性磷酸催化2-脱氧链烷胺的不对称糖基化及其在氨基糖苷合成中的应用
这项工作描述了手性磷酸(CPA)催化衍生自2-脱氧链胺的内消旋二醇的不对称糖基化。CPA的手性决定了糖基化反应的结果,在上述任何一种C4-糖基化中使用的对映体会计师结果(67
 :
: 33 DR)或C6-糖基化(86
33 DR)或C6-糖基化(86  :
: 14 DR)2- deoxystreptamines。这些糖基化的产物可以转化为氨基糖苷,并且描述了该策略在C4和C6位具有反向连接的保护的异亚胺和异卡那霉素B的合成中的应用。
14 DR)2- deoxystreptamines。这些糖基化的产物可以转化为氨基糖苷,并且描述了该策略在C4和C6位具有反向连接的保护的异亚胺和异卡那霉素B的合成中的应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号