当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic enantioselective aza-Diels–Alder reactions of unactivated acyclic 1,3-dienes with aryl-, alkenyl-, and alkyl-substituted imines
Chemical Communications ( IF 4.3 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1039/c7cc03010j Yasuo Hatanaka 1, 2, 3, 4 , Shuuto Nantaku 1, 2, 3, 4 , Yuhki Nishimura 1, 2, 3, 4 , Tomoyuki Otsuka 1, 2, 3, 4 , Tohru Sekikaw 1, 2, 3, 4
Chemical Communications ( IF 4.3 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1039/c7cc03010j Yasuo Hatanaka 1, 2, 3, 4 , Shuuto Nantaku 1, 2, 3, 4 , Yuhki Nishimura 1, 2, 3, 4 , Tomoyuki Otsuka 1, 2, 3, 4 , Tohru Sekikaw 1, 2, 3, 4
Affiliation
|
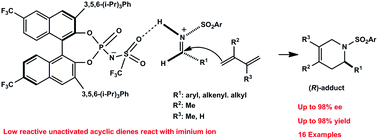
A catalytic enantioselective aza-Diels–Alder reaction of unactivated acyclic dienes with aryl-, alkenyl-, and alkyl-substituted imines is described. With 5–10 mol% loadings of a new Brønsted acid catalyst, the aza-Diels–Alder reaction of unactivated acyclic dienes proceeded to give the corresponding aza-Diels–Alder adducts in high yields (up to 98%) with excellent enantioselectivity (up to 98% ee). Preliminary DFT calculations suggest that the reaction proceeds through a chiral ion pair intermediate.
中文翻译:

未活化的无环1,3-二烯与芳基,烯基和烷基取代的亚胺的催化对映选择性氮杂-Diels-Alder反应
描述了未活化的无环二烯与芳基,烯基和烷基取代的亚胺的催化对映选择性氮杂-Diels-Alder反应。在新布朗斯台德酸催化剂的负载量为5-10 mol%的情况下,未活化的无环二烯的aza-Diels-Alder反应以高收率(高达98%)得到相应的aza-Diels-Alder加合物,具有出色的对映选择性(高达到98%ee)。初步的DFT计算表明该反应通过手性离子对中间体进行。
更新日期:2017-07-28
中文翻译:

未活化的无环1,3-二烯与芳基,烯基和烷基取代的亚胺的催化对映选择性氮杂-Diels-Alder反应
描述了未活化的无环二烯与芳基,烯基和烷基取代的亚胺的催化对映选择性氮杂-Diels-Alder反应。在新布朗斯台德酸催化剂的负载量为5-10 mol%的情况下,未活化的无环二烯的aza-Diels-Alder反应以高收率(高达98%)得到相应的aza-Diels-Alder加合物,具有出色的对映选择性(高达到98%ee)。初步的DFT计算表明该反应通过手性离子对中间体进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号