当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Guided Design and Initial Studies of a Bifunctional MEK/PI3K Inhibitor (ST-168)
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00111 Marcian E. Van Dort 1 , Stefanie Galbán 1 , Charles A. Nino 1 , Hao Hong 1 , April A. Apfelbaum 1 , Gary D. Luker 1 , Greg M. Thurber 1 , Lydia Atangcho 1 , Cagri G. Besirli 1 , Brian D. Ross 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00111 Marcian E. Van Dort 1 , Stefanie Galbán 1 , Charles A. Nino 1 , Hao Hong 1 , April A. Apfelbaum 1 , Gary D. Luker 1 , Greg M. Thurber 1 , Lydia Atangcho 1 , Cagri G. Besirli 1 , Brian D. Ross 1
Affiliation
|
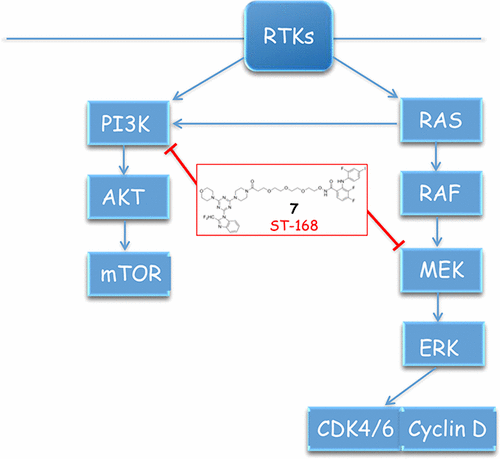
The structure-based design of a new single entity, MEK/PI3K bifunctional inhibitor (7, ST-168), which displays improved MEK1 and PI3K isoform inhibition, is described. ST-168 demonstrated a 2.2-fold improvement in MEK1 inhibition and a 2.8-, 2.7-, 23-, and 2.5-fold improved inhibition toward the PI3Kα, PI3Kβ, PI3Kδ, and PI3Kγ isoforms, respectively, as compared to a previous lead compound (4; ST-162) in in vitro enzymatic inhibition assays. ST-168 demonstrated superior tumoricidal efficacy over ST-162 in an A375 melanoma spheroid tumor model. ST-168 was comparatively more effective than ST-162 in promoting tumor control when administrated orally in a tumor therapy study conducted in an A375 melanoma mouse model confirming its bioavailability and efficacy toward combined in vivo MEK1/PI3K inhibition.
中文翻译:

双功能MEK / PI3K抑制剂的结构导向设计和初步研究(ST-168)
描述了一种新的单一实体MEK / PI3K双功能抑制剂(7,ST-168)的基于结构的设计,该抑制剂显示出改善的MEK1和PI3K同工型抑制作用。与以前的先导化合物相比,ST-168分别显示出对PI3Kα,PI3Kβ,PI3Kδ和PI3Kγ同工型的MEK1抑制作用提高了2.2倍,对PI3Kα,PI3Kβ,PI3Kδ和PI3Kγ的抑制作用分别提高了2.8、2.7、23和2.5倍。 (4;ST-162)在体外酶抑制试验中。在A375黑色素瘤球状肿瘤模型中,ST-168表现出优于ST-162的杀癌效果。ST-168比ST-162更有效在A375黑色素瘤小鼠模型中进行的肿瘤治疗研究中口服给药时,具有促进肿瘤控制的作用,证实了其对体内MEK1 / PI3K联合抑制的生物利用度和功效。
更新日期:2017-07-26
中文翻译:

双功能MEK / PI3K抑制剂的结构导向设计和初步研究(ST-168)
描述了一种新的单一实体MEK / PI3K双功能抑制剂(7,ST-168)的基于结构的设计,该抑制剂显示出改善的MEK1和PI3K同工型抑制作用。与以前的先导化合物相比,ST-168分别显示出对PI3Kα,PI3Kβ,PI3Kδ和PI3Kγ同工型的MEK1抑制作用提高了2.2倍,对PI3Kα,PI3Kβ,PI3Kδ和PI3Kγ的抑制作用分别提高了2.8、2.7、23和2.5倍。 (4;ST-162)在体外酶抑制试验中。在A375黑色素瘤球状肿瘤模型中,ST-168表现出优于ST-162的杀癌效果。ST-168比ST-162更有效在A375黑色素瘤小鼠模型中进行的肿瘤治疗研究中口服给药时,具有促进肿瘤控制的作用,证实了其对体内MEK1 / PI3K联合抑制的生物利用度和功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号