当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mutation of Conserved Residues Increases in Vitro Activity of the Formylglycine-Generating Enzyme
ChemBioChem ( IF 2.6 ) Pub Date : 2017-07-25 06:10:43 , DOI: 10.1002/cbic.201700174 Matthias Knop 1 , Roxana Lemnaru 1 , Florian P. Seebeck 1
ChemBioChem ( IF 2.6 ) Pub Date : 2017-07-25 06:10:43 , DOI: 10.1002/cbic.201700174 Matthias Knop 1 , Roxana Lemnaru 1 , Florian P. Seebeck 1
Affiliation
|
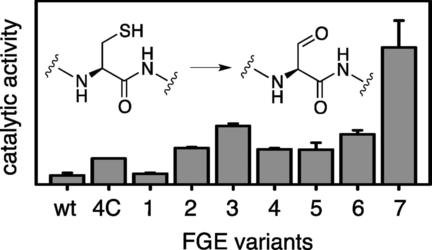
The formylglycine-generating enzyme (FGE) recognizes proteins with a specific cysteine-containing six-amino-acid motif and converts this cysteine residue into formylglycine. The resulting aldehyde function provides a unique handle for selective protein labeling. We have identified two mutations in FGE from Thermomonospora curvata that increase this catalytic efficiency more than 40-fold. The resulting activity and stability, as well as its ease of recombinant production, make this FGE variant a practical reagent for in vitro protein engineering.
中文翻译:

保守残基的突变增加了产生甲酰甘氨酸的酶的体外活性
甲酰甘氨酸生成酶(FGE)识别具有特定的含半胱氨酸的六氨基酸基序的蛋白质,并将该半胱氨酸残基转化为甲酰甘氨酸。所产生的醛功能为选择性蛋白质标记提供了独特的方法。我们已经从弯曲热单孢菌中鉴定出FGE中的两个突变,这些突变将这种催化效率提高了40倍以上。所产生的活性和稳定性以及其重组生产的简便性,使得该FGE变体成为用于体外蛋白质工程的实用试剂。
更新日期:2017-07-26
中文翻译:

保守残基的突变增加了产生甲酰甘氨酸的酶的体外活性
甲酰甘氨酸生成酶(FGE)识别具有特定的含半胱氨酸的六氨基酸基序的蛋白质,并将该半胱氨酸残基转化为甲酰甘氨酸。所产生的醛功能为选择性蛋白质标记提供了独特的方法。我们已经从弯曲热单孢菌中鉴定出FGE中的两个突变,这些突变将这种催化效率提高了40倍以上。所产生的活性和稳定性以及其重组生产的简便性,使得该FGE变体成为用于体外蛋白质工程的实用试剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号