当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclobutene vs 1,3-Diene Formation in the Gold-Catalyzed Reaction of Alkynes with Alkenes: The Complete Mechanistic Picture
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/jacs.7b03005 M. Elena de Orbe 1 , Laura Amenós 1 , Mariia S. Kirillova 1 , Yahui Wang 1 , Verónica López-Carrillo 1 , Feliu Maseras 1, 2 , Antonio M. Echavarren 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/jacs.7b03005 M. Elena de Orbe 1 , Laura Amenós 1 , Mariia S. Kirillova 1 , Yahui Wang 1 , Verónica López-Carrillo 1 , Feliu Maseras 1, 2 , Antonio M. Echavarren 1, 3
Affiliation
|
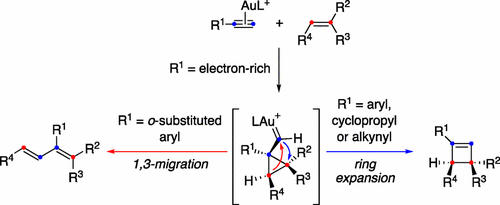
The intermolecular gold(I)-catalyzed reaction between arylalkynes and alkenes leads to cyclobutenes by a [2 + 2] cycloaddition, which takes place stepwise, first by formation of cyclopropyl gold(I) carbenes, followed by a ring expansion. However, 1,3-butadienes are also formed in the case of ortho-substituted arylalkynes by a metathesis-type process. The corresponding reaction of alkenes with aryl-1,3-butadiynes, ethynylogous to arylalkynes, leads exclusively to cyclobutenes. A comprehensive mechanism for the gold(I)-catalyzed reaction of alkynes with alkenes is proposed on the basis of density functional theory calculations, which shows that the two pathways leading to cyclobutenes or dienes are very close in energy. The key intermediates are cyclopropyl gold(I) carbenes, which have been independently generated by retro-Buchner reaction from stereodefined 1a,7b-dihydro-1H-cyclopropa[a]naphthalenes.
中文翻译:

炔烃与烯烃的金催化反应中环丁烯与1,3-二烯的形成:完整的机理图
芳基炔烃与烯烃之间的分子间金(I)催化反应通过[2 + 2]环加成反应逐步生成环丁烯,该过程逐步进行,首先形成环丙基金(I)卡宾,然后进行环扩环。但是,在邻位的情况下也会形成1,3-丁二烯复分解型方法取代了芳基炔烃。烯基与芳基炔烃对应的烯烃与芳基-1,3-丁二炔的相应反应仅产生环丁烯。在密度泛函理论计算的基础上,提出了炔烃与烯烃的金(I)催化反应的综合机理,表明生成环丁烯或二烯的两条途径的能量非常接近。关键中间体是环丙基金(I)卡宾,它们是通过逆向布赫纳反应从立体定义的1a,7b-二氢-1 H-环丙烷[ a ]萘独立生成的。
更新日期:2017-07-26
中文翻译:

炔烃与烯烃的金催化反应中环丁烯与1,3-二烯的形成:完整的机理图
芳基炔烃与烯烃之间的分子间金(I)催化反应通过[2 + 2]环加成反应逐步生成环丁烯,该过程逐步进行,首先形成环丙基金(I)卡宾,然后进行环扩环。但是,在邻位的情况下也会形成1,3-丁二烯复分解型方法取代了芳基炔烃。烯基与芳基炔烃对应的烯烃与芳基-1,3-丁二炔的相应反应仅产生环丁烯。在密度泛函理论计算的基础上,提出了炔烃与烯烃的金(I)催化反应的综合机理,表明生成环丁烯或二烯的两条途径的能量非常接近。关键中间体是环丙基金(I)卡宾,它们是通过逆向布赫纳反应从立体定义的1a,7b-二氢-1 H-环丙烷[ a ]萘独立生成的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号