当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Backbone Hydrogen Bond Strengths Can Vary Widely in Transmembrane Helices
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/jacs.7b04819 Zheng Cao 1 , James M. Hutchison 2 , Charles R. Sanders 2 , James U. Bowie 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/jacs.7b04819 Zheng Cao 1 , James M. Hutchison 2 , Charles R. Sanders 2 , James U. Bowie 1
Affiliation
|
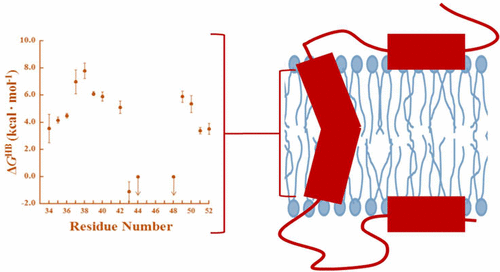
Although backbone hydrogen bonds in transmembrane (TM) helices have the potential to be very strong due to the low dielectric and low water environment of the membrane, their strength has never been assessed experimentally. Moreover, variations in hydrogen bond strength might be necessary to facilitate the TM helix breaking and bending that is often needed to satisfy functional imperatives. Here we employed equilibrium hydrogen/deuterium fractionation factors to measure backbone hydrogen bond strengths in the TM helix of the amyloid precursor protein (APP). We find an enormous range of hydrogen bond free energies, with some weaker than water–water hydrogen bonds and some over 6 kcal/mol stronger than water–water hydrogen bonds. We find that weak hydrogen bonds are at or near preferred γ-secretase cleavage sites, suggesting that the sequence of APP and possibly other cleaved TM helices may be designed, in part, to make their backbones accessible for cleavage. The finding that hydrogen bond strengths in a TM helix can vary widely has implications for membrane protein function, dynamics, evolution, and design.
中文翻译:

跨膜螺旋中的主链氢键强度可能会发生很大变化
尽管由于膜的低介电和低水环境,跨膜(TM)螺旋中的主链氢键具有非常强的潜力,但从未对它们的强度进行过实验评估。而且,可能需要改变氢键强度以促进TM螺旋的断裂和弯曲,这通常是满足功能要求所必需的。在这里,我们采用平衡氢/氘分馏因子来测量淀粉样前体蛋白(APP)的TM螺旋中的主链氢键强度。我们发现了范围广泛的氢键自由能,有些比水-水氢键弱,有些比水-水氢键强6 kcal / mol。我们发现弱氢键位于或靠近首选的γ-分泌酶裂解位点,提示APP和可能其他裂解的TM螺旋的序列可以部分地设计成使其骨架可被裂解。TM螺旋中氢键强度的差异可能很大,这一发现对膜蛋白的功能,动力学,进化和设计都具有重要意义。
更新日期:2017-07-26
中文翻译:

跨膜螺旋中的主链氢键强度可能会发生很大变化
尽管由于膜的低介电和低水环境,跨膜(TM)螺旋中的主链氢键具有非常强的潜力,但从未对它们的强度进行过实验评估。而且,可能需要改变氢键强度以促进TM螺旋的断裂和弯曲,这通常是满足功能要求所必需的。在这里,我们采用平衡氢/氘分馏因子来测量淀粉样前体蛋白(APP)的TM螺旋中的主链氢键强度。我们发现了范围广泛的氢键自由能,有些比水-水氢键弱,有些比水-水氢键强6 kcal / mol。我们发现弱氢键位于或靠近首选的γ-分泌酶裂解位点,提示APP和可能其他裂解的TM螺旋的序列可以部分地设计成使其骨架可被裂解。TM螺旋中氢键强度的差异可能很大,这一发现对膜蛋白的功能,动力学,进化和设计都具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号