当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Directed β C–H Amination of Alcohols via Radical Relay Chaperones
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-25 , DOI: 10.1021/jacs.7b05214 Ethan A Wappes 1 , Kohki M Nakafuku 1 , David A Nagib 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-25 , DOI: 10.1021/jacs.7b05214 Ethan A Wappes 1 , Kohki M Nakafuku 1 , David A Nagib 1
Affiliation
|
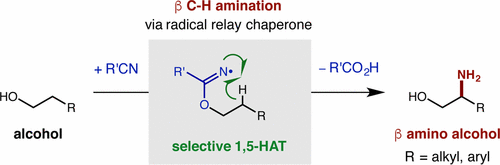
A radical-mediated strategy for β C-H amination of alcohols has been developed. This approach employs a radical relay chaperone, which serves as a traceless director that facilitates selective C-H functionalization via 1,5-hydrogen atom transfer (HAT) and enables net incorporation of ammonia at the β carbon of alcohols. The chaperones presented herein enable direct access to imidate radicals, allowing their first use for H atom abstraction. A streamlined protocol enables rapid conversion of alcohols to their β-amino analogs (via in situ conversion of alcohols to imidates, directed C-H amination, and hydrolysis to NH2). Mechanistic experiments indicate HAT is rate-limiting, whereas intramolecular amination is product- and stereo-determining.
中文翻译:

通过自由基中继伴侣对醇进行定向 β C–H 胺化
已经开发出一种自由基介导的醇 β CH 胺化策略。该方法采用自由基中继伴侣,作为无痕导向器,通过 1,5-氢原子转移 (HAT) 促进选择性 CH 官能化,并能够在醇的 β 碳上净掺入氨。本文提出的分子伴侣能够直接接触酰亚胺基团,从而允许它们首次用于 H 原子的提取。简化的方案能够将醇快速转化为其 β-氨基类似物(通过将醇原位转化为亚氨酸酯、直接 CH 胺化和水解为 NH2)。机理实验表明 HAT 是限速的,而分子内胺化是产物和立体决定的。
更新日期:2017-07-25
中文翻译:

通过自由基中继伴侣对醇进行定向 β C–H 胺化
已经开发出一种自由基介导的醇 β CH 胺化策略。该方法采用自由基中继伴侣,作为无痕导向器,通过 1,5-氢原子转移 (HAT) 促进选择性 CH 官能化,并能够在醇的 β 碳上净掺入氨。本文提出的分子伴侣能够直接接触酰亚胺基团,从而允许它们首次用于 H 原子的提取。简化的方案能够将醇快速转化为其 β-氨基类似物(通过将醇原位转化为亚氨酸酯、直接 CH 胺化和水解为 NH2)。机理实验表明 HAT 是限速的,而分子内胺化是产物和立体决定的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号