当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
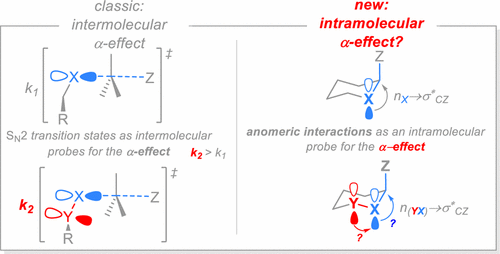
Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular α-Effect
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/jacs.7b05367 Eusebio Juaristi 1, 2 , Gabriel dos Passos Gomes 3 , Alexander O. Terent’ev 4 , Rafael Notario 5 , Igor V. Alabugin 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/jacs.7b05367 Eusebio Juaristi 1, 2 , Gabriel dos Passos Gomes 3 , Alexander O. Terent’ev 4 , Rafael Notario 5 , Igor V. Alabugin 3
Affiliation
|
The first systematic study of the intramolecular α-effect, both in the stable ground-state structures and in the high-energy intermediates, was accomplished using the anomeric effect as an internal stereoelectronic probe. Contrary to the expectations based on the simple orbital mixing model, the lone pairs in a pair of neutral directly connected heteroatoms are not raised in energy to become stronger donors toward adjacent σ- and π-acceptors. Instead, the key n(X-Y)→σ*C-F interactions (X,Y = O,N) in the “α-systems” (both acyclic and constrained within a heterocyclohexane frame) are weaker than nX→σ*C-F interactions in “normal” systems. Surprisingly, polar solvent effects increase the apparent magnitude of α-effect as measured via increase in the anomeric stabilization. This behavior is opposite to the solvent dependence of normal systems where the anomeric effect is severely weakened by polar solvents. This contrasting behavior reflects the different balance of electrostatic and conjugative interactions in the two types of anomeric systems: the α-systems suffer less from the unfavorable orientation of bond dipoles in the equatorial conformer, a destabilizing electrostatic effect that is shielded by the polar environments. A weak α-effect is brought to life when the buttressing α-heteroatom bears a negative charge. However, electrostatic components mask the role of stabilizing orbital interactions. In contrast, the increased electron demand in carbocations and related electron-deficient TS- like structures does not lead to activation of the α-effect. As a consequence, we observed that ethers are better radical- and cation-stabilizing groups than peroxides. The latter finding should have significant implications for understanding the mechanistic complexity associated with the interaction of carbonyl compounds with hydroperoxides and H2O2 in acidic media, as such reactions involve α-cationic intermediates.
中文翻译:

立体电子相互作用作为分子内α效应存在的探针
使用异头作用作为内部立体电子探针,对稳定的基态结构和高能中间体中的分子内α效应进行了首次系统研究。与基于简单轨道混合模型的预期相反,一对中性直接连接的杂原子中的孤对并未提高能量,从而成为朝向相邻σ和π受体的更强供体。取而代之的是,“α系统”中的键n (XY) →σ* CF相互作用(X,Y = O,N)(无环且受杂环己烷的约束)比n X →σ* CF弱“正常”系统中的互动。出乎意料的是,通过增加端基异构化稳定性,极性溶剂效应增加了α-效应的表观幅度。此行为与常规系统的溶剂依赖性相反,在常规系统中,极性溶剂会严重削弱端基异构作用。这种相反的行为反映出两种类型的异头异构体系统在静电和共轭相互作用方面的不同平衡:α系统受赤道构象体中键合偶极子不利取向的影响较小,这种不稳定的静电作用受到极性环境的屏蔽。当支撑的α-杂原子带有负电荷时,会产生微弱的α效应。但是,静电成分掩盖了稳定轨道相互作用的作用。相比之下,类似的结构不会导致α效应的激活。结果,我们观察到醚是比过氧化物更好的自由基和阳离子稳定基团。后一发现对于理解与羰基化合物与氢过氧化物和H 2 O 2在酸性介质中相互作用有关的机理复杂性具有重要意义,因为这种反应涉及α-阳离子中间体。
更新日期:2017-07-26
中文翻译:

立体电子相互作用作为分子内α效应存在的探针
使用异头作用作为内部立体电子探针,对稳定的基态结构和高能中间体中的分子内α效应进行了首次系统研究。与基于简单轨道混合模型的预期相反,一对中性直接连接的杂原子中的孤对并未提高能量,从而成为朝向相邻σ和π受体的更强供体。取而代之的是,“α系统”中的键n (XY) →σ* CF相互作用(X,Y = O,N)(无环且受杂环己烷的约束)比n X →σ* CF弱“正常”系统中的互动。出乎意料的是,通过增加端基异构化稳定性,极性溶剂效应增加了α-效应的表观幅度。此行为与常规系统的溶剂依赖性相反,在常规系统中,极性溶剂会严重削弱端基异构作用。这种相反的行为反映出两种类型的异头异构体系统在静电和共轭相互作用方面的不同平衡:α系统受赤道构象体中键合偶极子不利取向的影响较小,这种不稳定的静电作用受到极性环境的屏蔽。当支撑的α-杂原子带有负电荷时,会产生微弱的α效应。但是,静电成分掩盖了稳定轨道相互作用的作用。相比之下,类似的结构不会导致α效应的激活。结果,我们观察到醚是比过氧化物更好的自由基和阳离子稳定基团。后一发现对于理解与羰基化合物与氢过氧化物和H 2 O 2在酸性介质中相互作用有关的机理复杂性具有重要意义,因为这种反应涉及α-阳离子中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号