当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Hydrogen Bonding Catalysis for the Synthesis of Dihydroquinazoline-Containing Antiviral, Letermovir
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/jacs.7b05806 Cheol K. Chung 1 , Zhijian Liu 1 , Katrina W. Lexa 1 , Teresa Andreani 1 , Yingju Xu 1 , Yining Ji 1 , Daniel A. DiRocco 1 , Guy R. Humphrey 1 , Rebecca T. Ruck 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/jacs.7b05806 Cheol K. Chung 1 , Zhijian Liu 1 , Katrina W. Lexa 1 , Teresa Andreani 1 , Yingju Xu 1 , Yining Ji 1 , Daniel A. DiRocco 1 , Guy R. Humphrey 1 , Rebecca T. Ruck 1
Affiliation
|
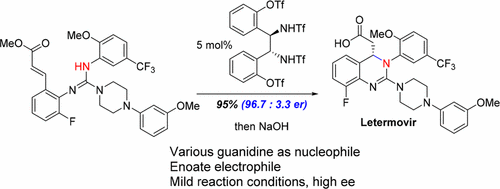
A weak Brønsted acid-catalyzed asymmetric guanidine aza-conjugate addition reaction has been developed. C2-symmetric, dual hydrogen-bond donating bistriflamides are shown to be highly effective in activating α,β-unsaturated esters toward the intramolecular addition of a pendant guanidinyl nucleophile. Preliminary mechanistic investigation, including density functional theory calculations and kinetics studies, support a conjugate addition pathway as more favorable energetically than an alternative electrocyclization pathway. This methodology has been successfully applied to the synthesis of the 3,4-dihydroquinazoline-containing antiviral, Letermovir, and a series of analogues.
中文翻译:

不对称氢键催化合成含二氢喹唑啉类抗病毒药物,来特莫韦
已经开发出弱的布朗斯台德酸催化的不对称胍氮杂-共轭加成反应。C 2对称的双氢键供体双三氟化物被证明在激活α,β-不饱和酯朝着侧基胍基亲核分子的分子内加成方面非常有效。初步的机械研究,包括密度泛函理论计算和动力学研究,都认为共轭加成途径比替代性电环化途径在能量上更有利。该方法已成功应用于含3,4-二氢喹唑啉的抗病毒药物,来特莫韦和一系列类似物的合成。
更新日期:2017-07-26
中文翻译:

不对称氢键催化合成含二氢喹唑啉类抗病毒药物,来特莫韦
已经开发出弱的布朗斯台德酸催化的不对称胍氮杂-共轭加成反应。C 2对称的双氢键供体双三氟化物被证明在激活α,β-不饱和酯朝着侧基胍基亲核分子的分子内加成方面非常有效。初步的机械研究,包括密度泛函理论计算和动力学研究,都认为共轭加成途径比替代性电环化途径在能量上更有利。该方法已成功应用于含3,4-二氢喹唑啉的抗病毒药物,来特莫韦和一系列类似物的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号