当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FRET monitoring of a nonribosomal peptide synthetase
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-07-24 00:00:00 , DOI: 10.1038/nchembio.2435 Jonas Alfermann , Xun Sun , Florian Mayerthaler , Thomas E Morrell , Eva Dehling , Gerrit Volkmann , Tamiki Komatsuzaki , Haw Yang , Henning D Mootz
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-07-24 00:00:00 , DOI: 10.1038/nchembio.2435 Jonas Alfermann , Xun Sun , Florian Mayerthaler , Thomas E Morrell , Eva Dehling , Gerrit Volkmann , Tamiki Komatsuzaki , Haw Yang , Henning D Mootz
|
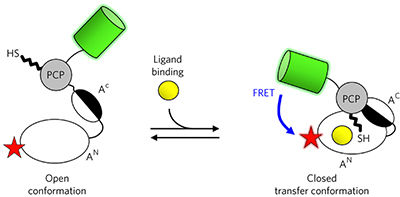
Nonribosomal peptide synthetases (NRPSs) are multidomain enzyme templates for the synthesis of bioactive peptides. Large-scale conformational changes during peptide assembly are obvious from crystal structures, yet their dynamics and coupling to catalysis are poorly understood. We have designed an NRPS FRET sensor to monitor, in solution and in real time, the adoption of the productive transfer conformation between phenylalanine-binding adenylation (A) and peptidyl-carrier-protein domains of gramicidin synthetase I from Aneurinibacillus migulanus. The presence of ligands, substrates or intermediates induced a distinct fluorescence resonance energy transfer (FRET) readout, which was pinpointed to the population of specific conformations or, in two cases, mixtures of conformations. A pyrophosphate switch and lysine charge sensors control the domain alternation of the A domain. The phenylalanine–thioester and phenylalanine–AMP products constitute a mechanism of product inhibition and release that is involved in ordered assembly-line peptide biosynthesis. Our results represent insights from solution measurements into the conformational dynamics of the catalytic cycle of NRPSs.
中文翻译:

FRET监测非核糖体肽合成酶
非核糖体肽合成酶(NRPS)是用于合成生物活性肽的多域酶模板。从晶体结构来看,肽组装过程中的大规模构象变化是显而易见的,但人们对它们的动力学以及与催化的耦合了解甚少。我们设计了一种NRPS FRET传感器,用于实时监测溶液中米亚圭尼罗星杆菌合成酶I的苯丙氨酸结合腺苷酸化(A)和肽基载体蛋白结构域之间的生产转移构象的采用。配体,底物或中间体的存在引发了明显的荧光共振能量转移(FRET)读数,该读数被精确定位为特定构象的群体,或者在两种情况下为构象的混合物。焦磷酸盐开关和赖氨酸电荷传感器控制A域的域交替。苯丙氨酸-硫酯和苯丙氨酸-AMP产物构成产物抑制和释放的机制,参与有序组装线肽生物合成。我们的结果代表了从溶液测量到NRPS催化循环构象动力学的见解。
更新日期:2017-07-25
中文翻译:

FRET监测非核糖体肽合成酶
非核糖体肽合成酶(NRPS)是用于合成生物活性肽的多域酶模板。从晶体结构来看,肽组装过程中的大规模构象变化是显而易见的,但人们对它们的动力学以及与催化的耦合了解甚少。我们设计了一种NRPS FRET传感器,用于实时监测溶液中米亚圭尼罗星杆菌合成酶I的苯丙氨酸结合腺苷酸化(A)和肽基载体蛋白结构域之间的生产转移构象的采用。配体,底物或中间体的存在引发了明显的荧光共振能量转移(FRET)读数,该读数被精确定位为特定构象的群体,或者在两种情况下为构象的混合物。焦磷酸盐开关和赖氨酸电荷传感器控制A域的域交替。苯丙氨酸-硫酯和苯丙氨酸-AMP产物构成产物抑制和释放的机制,参与有序组装线肽生物合成。我们的结果代表了从溶液测量到NRPS催化循环构象动力学的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号