当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Nothing Boosts Affinity: Hydrophobic Ligand Binding to the Virtually Vacated S1′ Pocket of Thermolysin
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-24 00:00:00 , DOI: 10.1021/jacs.7b05028 Stefan G. Krimmer 1 , Jonathan Cramer 1 , Johannes Schiebel 1 , Andreas Heine 1 , Gerhard Klebe 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-24 00:00:00 , DOI: 10.1021/jacs.7b05028 Stefan G. Krimmer 1 , Jonathan Cramer 1 , Johannes Schiebel 1 , Andreas Heine 1 , Gerhard Klebe 1
Affiliation
|
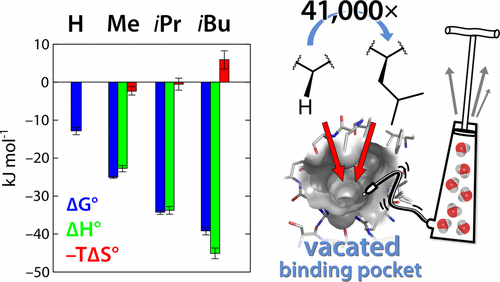
We investigated the hydration state of the deep, well-accessible hydrophobic S1′ specificity pocket of the metalloprotease thermolysin with purposefully designed ligands using high-resolution crystallography and isothermal titration calorimetry. The S1′ pocket is known to recognize selectively a very stringent set of aliphatic side chains such as valine, leucine, and isoleucine of putative substrates. We engineered a weak-binding ligand covering the active site of the protease without addressing the S1′ pocket, thus transforming it into an enclosed cavity. Its sustained accessibility could be proved by accommodating noble gas atoms into the pocket in the crystalline state. The topology and electron content of the enclosed pocket with a volume of 141 Å3 were analyzed using an experimental MAD-phased electron density map that was calibrated to an absolute electron number scale, enabling access to the total electron content within the cavity. Our analysis indicates that the S1′ pocket is virtually vacated, thus free of any water molecules. The thermodynamic signature of the reduction of the void within the pocket by growing aliphatic P1′ substituents (H, Me, iPr, iBu) reveals a dramatic, enthalpy-dominated gain in free energy of binding resulting in a factor of 41 000 in Kd for the H-to-iBu transformation. Substituents placing polar decoy groups into the pocket to capture putatively present water molecules could not collect any evidence for a bound solvent molecule.
中文翻译:

没有什么能增强亲和力:疏水配体结合到实际上空洞的嗜热菌素S 1 '口袋中
我们使用高分辨率的晶体学和等温滴定量热法研究了金属蛋白酶嗜热菌素的深层,易于接近的疏水性S 1 '特异性口袋的水合状态,该口袋有针对性地设计了配体。已知S 1 '口袋选择性地识别非常严格的一组脂族侧链,例如推定底物的缬氨酸,亮氨酸和异亮氨酸。我们设计了一种弱结合配体,其覆盖了蛋白酶的活性位点,而没有解决S 1 '口袋,因此将其转化为封闭的空腔。通过将稀有气体原子以结晶状态容纳在袋中,可以证明其持久的可及性。体积为141Å的密闭型腔的拓扑结构和电子含量使用实验的MAD相电子密度图分析了图3中的电子密度图,该图已校准为绝对电子数标度,从而可以访问腔体内的总电子含量。我们的分析表明,S 1 '口袋实际上是空的,因此没有任何水分子。通过生长脂族P 1 '取代基(H,Me,i Pr,i Bu)减少口袋中空隙的热力学特征表明,结合自由能显着地以焓为主,增加了41 000倍在ķ d为H-TO-我卜变换。将极性诱饵基团放到口袋中以捕获假定存在的水分子的替代物无法收集到有关结合的溶剂分子的任何证据。
更新日期:2017-07-25
中文翻译:

没有什么能增强亲和力:疏水配体结合到实际上空洞的嗜热菌素S 1 '口袋中
我们使用高分辨率的晶体学和等温滴定量热法研究了金属蛋白酶嗜热菌素的深层,易于接近的疏水性S 1 '特异性口袋的水合状态,该口袋有针对性地设计了配体。已知S 1 '口袋选择性地识别非常严格的一组脂族侧链,例如推定底物的缬氨酸,亮氨酸和异亮氨酸。我们设计了一种弱结合配体,其覆盖了蛋白酶的活性位点,而没有解决S 1 '口袋,因此将其转化为封闭的空腔。通过将稀有气体原子以结晶状态容纳在袋中,可以证明其持久的可及性。体积为141Å的密闭型腔的拓扑结构和电子含量使用实验的MAD相电子密度图分析了图3中的电子密度图,该图已校准为绝对电子数标度,从而可以访问腔体内的总电子含量。我们的分析表明,S 1 '口袋实际上是空的,因此没有任何水分子。通过生长脂族P 1 '取代基(H,Me,i Pr,i Bu)减少口袋中空隙的热力学特征表明,结合自由能显着地以焓为主,增加了41 000倍在ķ d为H-TO-我卜变换。将极性诱饵基团放到口袋中以捕获假定存在的水分子的替代物无法收集到有关结合的溶剂分子的任何证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号