当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Total Synthesis of Nigelladine A via Late-Stage C–H Oxidation Enabled by an Engineered P450 Enzyme
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-24 00:00:00 , DOI: 10.1021/jacs.7b05196 Steven A. Loskot 1 , David K. Romney 1 , Frances H. Arnold 1 , Brian M. Stoltz 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-24 00:00:00 , DOI: 10.1021/jacs.7b05196 Steven A. Loskot 1 , David K. Romney 1 , Frances H. Arnold 1 , Brian M. Stoltz 1
Affiliation
|
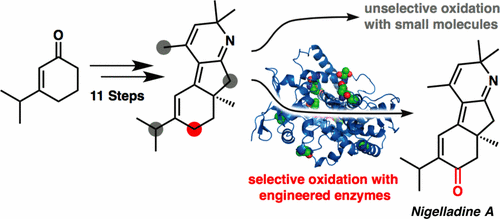
An enantioselective total synthesis of the norditerpenoid alkaloid nigelladine A is described. Strategically, the synthesis relies on a late-stage C–H oxidation of an advanced intermediate. While traditional chemical methods failed to deliver the desired outcome, an engineered cytochrome P450 enzyme was employed to effect a chemo- and regioselective allylic C–H oxidation in the presence of four oxidizable positions. The enzyme variant was readily identified from a focused library of three enzymes, allowing for completion of the synthesis without the need for extensive screening.
中文翻译:

通过工程化的P450酶促成的后期C–H氧化对尼古拉定A的对映选择性全合成
描述了对二萜类生物碱尼古拉定A的对映选择性全合成。从战略上讲,该合成依赖于高级中间体的后期C–H氧化。尽管传统的化学方法无法提供理想的结果,但工程化的细胞色素P450酶在存在四个可氧化位置的情况下,实现了化学和区域选择性烯丙基CH的氧化。可从三种酶的集中文库中轻松鉴定出酶变体,从而无需大量筛选即可完成合成。
更新日期:2017-07-25
中文翻译:

通过工程化的P450酶促成的后期C–H氧化对尼古拉定A的对映选择性全合成
描述了对二萜类生物碱尼古拉定A的对映选择性全合成。从战略上讲,该合成依赖于高级中间体的后期C–H氧化。尽管传统的化学方法无法提供理想的结果,但工程化的细胞色素P450酶在存在四个可氧化位置的情况下,实现了化学和区域选择性烯丙基CH的氧化。可从三种酶的集中文库中轻松鉴定出酶变体,从而无需大量筛选即可完成合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号