当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Borylenes: An Emerging Class of Compounds
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-21 05:00:43 , DOI: 10.1002/anie.201705153 Michele Soleilhavoup 1 , Guy Bertrand 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-21 05:00:43 , DOI: 10.1002/anie.201705153 Michele Soleilhavoup 1 , Guy Bertrand 1
Affiliation
|
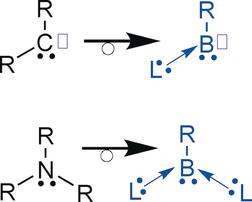
Free borylenes (R–B:) have only been spectroscopically characterized in the gas phase or in matrices at very low temperatures. However, in recent years, a few mono- and bis(Lewis base)-stabilized borylenes have been isolated. In both of these compounds the boron atom is in the formal oxidation state +I which contrasts with classical organoboron derivatives wherein the element is in the +III oxidation state. Mono(Lewis base)-stabilized borylenes are isoelectronic with singlet carbenes, and their reactivity mimics to some extent that of transition metals. They can activate small molecules, such as H2, and coordinate an additional ligand; in other words, they are boron metallomimics. Bis(Lewis base)borylene adducts are isoelectronic with amines and phosphines. In contrast to boranes, which act as electron acceptors and thus Lewis acids, they are electron-rich and act as ligands for transition metals.
中文翻译:

硼烯:一类新兴的化合物
游离硼烷(RB :)仅在气相中或在非常低的温度下在基质中进行了光谱表征。然而,近年来,已经分离出一些单和双(路易斯碱)稳定的亚芳基。在这两种化合物中,硼原子均处于形式氧化态+ I,这与经典的有机硼衍生物形成对比,在传统的有机硼衍生物中,元素处于+ III氧化态。单(刘易斯碱)稳定的亚芳基与单线碳烯是等电子的,它们的反应性在某种程度上模仿了过渡金属。它们可以激活小分子,例如H 2,并协调其他配体;换句话说,它们是硼金属化学。双(路易斯碱)甲硼烷加合物与胺和膦等电子。与硼烷不同,硼烷充当电子受体,因此是路易斯酸,它们富含电子,并充当过渡金属的配体。
更新日期:2017-07-22
中文翻译:

硼烯:一类新兴的化合物
游离硼烷(RB :)仅在气相中或在非常低的温度下在基质中进行了光谱表征。然而,近年来,已经分离出一些单和双(路易斯碱)稳定的亚芳基。在这两种化合物中,硼原子均处于形式氧化态+ I,这与经典的有机硼衍生物形成对比,在传统的有机硼衍生物中,元素处于+ III氧化态。单(刘易斯碱)稳定的亚芳基与单线碳烯是等电子的,它们的反应性在某种程度上模仿了过渡金属。它们可以激活小分子,例如H 2,并协调其他配体;换句话说,它们是硼金属化学。双(路易斯碱)甲硼烷加合物与胺和膦等电子。与硼烷不同,硼烷充当电子受体,因此是路易斯酸,它们富含电子,并充当过渡金属的配体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号