当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Metal Drives the Chemistry: Dual Functions of Acireductone Dioxygenase
Chemical Reviews ( IF 51.4 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/acs.chemrev.7b00117 Aditi R. Deshpande 1 , Thomas C. Pochapsky 1 , Dagmar Ringe 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/acs.chemrev.7b00117 Aditi R. Deshpande 1 , Thomas C. Pochapsky 1 , Dagmar Ringe 1
Affiliation
|
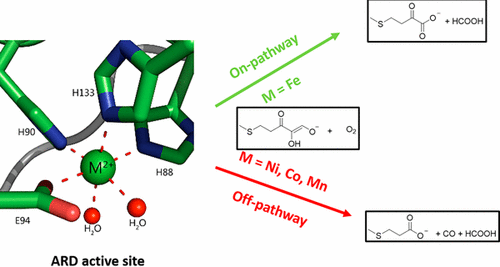
Acireductone dioxygenase (ARD) from the methionine salvage pathway (MSP) is a unique enzyme that exhibits dual chemistry determined solely by the identity of the divalent transition-metal ion (Fe2+ or Ni2+) in the active site. The Fe2+-containing isozyme catalyzes the on-pathway reaction using substrates 1,2-dihydroxy-3-keto-5-methylthiopent-1-ene (acireductone) and dioxygen to generate formate and the ketoacid precursor of methionine, 2-keto-4-methylthiobutyrate, whereas the Ni2+-containing isozyme catalyzes an off-pathway shunt with the same substrates, generating methylthiopropionate, carbon monoxide, and formate. The dual chemistry of ARD was originally discovered in the bacterium Klebsiella oxytoca, but it has recently been shown that mammalian ARD enzymes (mouse and human) are also capable of catalyzing metal-dependent dual chemistry in vitro. This is particularly interesting, since carbon monoxide, one of the products of off-pathway reaction, has been identified as an antiapoptotic molecule in mammals. In addition, several biochemical and genetic studies have indicated an inhibitory role of human ARD in cancer. This comprehensive review describes the biochemical and structural characterization of the ARD family, the proposed experimental and theoretical approaches to establishing mechanisms for the dual chemistry, insights into the mechanism based on comparison with structurally and functionally similar enzymes, and the applications of this research to the field of artificial metalloenzymes and synthetic biology.
中文翻译:

金属推动化学反应:乙炔基双加氧酶的双重功能
来自甲硫氨酸抢救途径(MSP)的乙酰丙二酮双加氧酶(ARD)是一种独特的酶,具有双重化学作用,该化学作用仅由活性位点中的二价过渡金属离子(Fe 2+或Ni 2+)的身份决定。含Fe 2+的同工酶使用底物1,2-二羟基-3-酮基-5-甲基硫喷烯-1-烯(丙烯醛)和双氧催化生成途中的甲酸酯和蛋氨酸,2-酮的酮酸前体-4-甲基硫代丁酸,而含Ni 2+的同工酶以相同的底物催化离路分流,产生甲基硫代丙酸,一氧化碳和甲酸盐。ARD的双重化学物质最初是在产酸克雷伯菌中发现的,但最近发现哺乳动物ARD酶(小鼠和人类)也能够在体外催化金属依赖性双重化学反应。这是特别令人感兴趣的,因为一氧化碳是一种非通路反应的产物,已被确定为哺乳动物中的一种抗凋亡分子。另外,一些生化和遗传研究表明人ARD在癌症中具有抑制作用。这项全面的综述描述了ARD家族的生化和结构特征,提议的建立双化学机理的实验和理论方法,基于与结构和功能相似的酶的比较对机理的深刻见解以及该研究在核酸中的应用金属酶和合成生物学领域。
更新日期:2017-07-21
中文翻译:

金属推动化学反应:乙炔基双加氧酶的双重功能
来自甲硫氨酸抢救途径(MSP)的乙酰丙二酮双加氧酶(ARD)是一种独特的酶,具有双重化学作用,该化学作用仅由活性位点中的二价过渡金属离子(Fe 2+或Ni 2+)的身份决定。含Fe 2+的同工酶使用底物1,2-二羟基-3-酮基-5-甲基硫喷烯-1-烯(丙烯醛)和双氧催化生成途中的甲酸酯和蛋氨酸,2-酮的酮酸前体-4-甲基硫代丁酸,而含Ni 2+的同工酶以相同的底物催化离路分流,产生甲基硫代丙酸,一氧化碳和甲酸盐。ARD的双重化学物质最初是在产酸克雷伯菌中发现的,但最近发现哺乳动物ARD酶(小鼠和人类)也能够在体外催化金属依赖性双重化学反应。这是特别令人感兴趣的,因为一氧化碳是一种非通路反应的产物,已被确定为哺乳动物中的一种抗凋亡分子。另外,一些生化和遗传研究表明人ARD在癌症中具有抑制作用。这项全面的综述描述了ARD家族的生化和结构特征,提议的建立双化学机理的实验和理论方法,基于与结构和功能相似的酶的比较对机理的深刻见解以及该研究在核酸中的应用金属酶和合成生物学领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号