当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Steroidogenic Metabolism of Galeterone Reveals a Diversity of Biochemical Activities
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2017-06-22 , DOI: 10.1016/j.chembiol.2017.05.020 Mohammad Alyamani 1 , Zhenfei Li 2 , Michael Berk 2 , Jianneng Li 2 , Jingjie Tang 3 , Sunil Upadhyay 4 , Richard J Auchus 4 , Nima Sharifi 5
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2017-06-22 , DOI: 10.1016/j.chembiol.2017.05.020 Mohammad Alyamani 1 , Zhenfei Li 2 , Michael Berk 2 , Jianneng Li 2 , Jingjie Tang 3 , Sunil Upadhyay 4 , Richard J Auchus 4 , Nima Sharifi 5
Affiliation
|
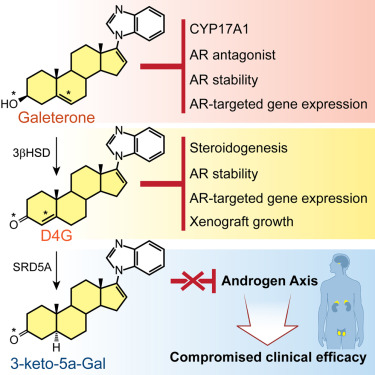
Galeterone is a steroidal CYP17A1 inhibitor, androgen receptor (AR) antagonist, and AR degrader, under evaluation in a phase III clinical trial for castration-resistant prostate cancer (CRPC). The A/B steroid ring (Δ5,3β-hydroxyl) structure of galeterone is identical to that of cholesterol, which makes endogenous steroids with the same structure (e.g., dehydroepiandrosterone and pregnenolone) substrates for the enzyme 3β-hydroxysteroid dehydrogenase (3βHSD). We found that galeterone is metabolized by 3βHSD to Δ4-galeterone (D4G), which is further converted by steroid-5α-reductase (SRD5A) to 3-keto-5α-galeterone (5αG), 3α-OH-5α-galeterone, and 3β-OH-5α-galeterone; in vivo it is also converted to the three corresponding 5β-reduced metabolites. D4G inhibits steroidogenesis and suppresses AR protein stability, AR target gene expression, and xenograft growth comparably with galeterone, and further conversion by SRD5A leads to loss of several activities that inhibit the androgen axis that may compromise clinical efficacy. Together, these findings define a critical metabolic class effect of steroidal drugs with a Δ5,3β-hydroxyl structure.
中文翻译:

加莱特酮的类固醇代谢揭示了生化活性的多样性
Galeterone 是一种类固醇 CYP17A1 抑制剂、雄激素受体 (AR) 拮抗剂和 AR 降解剂,正在针对去势抵抗性前列腺癌 (CRPC) 的 III 期临床试验中进行评估。伽莱特酮的 A/B 类固醇环(Δ5,3β-羟基)结构与胆固醇相同,这使得具有相同结构的内源性类固醇(例如脱氢表雄酮和孕烯醇酮)成为 3β-羟基类固醇脱氢酶(3βHSD)的底物。我们发现,伽莱特酮被 3βHSD 代谢为 Δ4-伽莱特酮 (D4G),其进一步被类固醇 5α-还原酶 (SRD5A) 转化为 3-酮-5α-伽莱特酮 (5αG)、3α-OH-5α-伽莱特酮,以及3β-OH-5α-伽莱特酮;在体内它也转化为三种相应的 5β-还原代谢物。与伽莱特酮相比,D4G 抑制类固醇生成并抑制 AR 蛋白稳定性、AR 靶基因表达和异种移植物生长,并且 SRD5A 的进一步转化导致一些抑制雄激素轴的活性丧失,从而可能损害临床疗效。总之,这些发现定义了具有 Δ5,3β-羟基结构的类固醇药物的关键代谢类效应。
更新日期:2017-07-22
中文翻译:

加莱特酮的类固醇代谢揭示了生化活性的多样性
Galeterone 是一种类固醇 CYP17A1 抑制剂、雄激素受体 (AR) 拮抗剂和 AR 降解剂,正在针对去势抵抗性前列腺癌 (CRPC) 的 III 期临床试验中进行评估。伽莱特酮的 A/B 类固醇环(Δ5,3β-羟基)结构与胆固醇相同,这使得具有相同结构的内源性类固醇(例如脱氢表雄酮和孕烯醇酮)成为 3β-羟基类固醇脱氢酶(3βHSD)的底物。我们发现,伽莱特酮被 3βHSD 代谢为 Δ4-伽莱特酮 (D4G),其进一步被类固醇 5α-还原酶 (SRD5A) 转化为 3-酮-5α-伽莱特酮 (5αG)、3α-OH-5α-伽莱特酮,以及3β-OH-5α-伽莱特酮;在体内它也转化为三种相应的 5β-还原代谢物。与伽莱特酮相比,D4G 抑制类固醇生成并抑制 AR 蛋白稳定性、AR 靶基因表达和异种移植物生长,并且 SRD5A 的进一步转化导致一些抑制雄激素轴的活性丧失,从而可能损害临床疗效。总之,这些发现定义了具有 Δ5,3β-羟基结构的类固醇药物的关键代谢类效应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号