当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Attenuating Staphylococcus aureus Virulence by Targeting Flotillin Protein Scaffold Activity
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2017-06-29 , DOI: 10.1016/j.chembiol.2017.05.027 Gudrun Koch 1 , Charlotte Wermser 1 , Ivan C Acosta 2 , Lara Kricks 2 , Stephanie T Stengel 1 , Ana Yepes 1 , Daniel Lopez 3
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2017-06-29 , DOI: 10.1016/j.chembiol.2017.05.027 Gudrun Koch 1 , Charlotte Wermser 1 , Ivan C Acosta 2 , Lara Kricks 2 , Stephanie T Stengel 1 , Ana Yepes 1 , Daniel Lopez 3
Affiliation
|
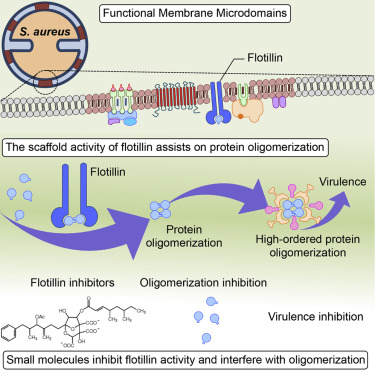
Scaffold proteins are ubiquitous chaperones that bind proteins and facilitate physical interaction of multi-enzyme complexes. Here we used a biochemical approach to dissect the scaffold activity of the flotillin-homolog protein FloA of the multi-drug-resistant human pathogenStaphylococcus aureus. We show that FloA promotes oligomerization of membrane protein complexes, such as the membrane-associated RNase Rny, which forms part of the RNA-degradation machinery called the degradosome. Cells lacking FloA had reduced Rny function and a consequent increase in the targeted sRNA transcripts that negatively regulateS. aureustoxin expression. Small molecules that altered FloA oligomerization also reduced Rny function and decreased the virulence potential ofS. aureusin vitro, as well as in vivo, using invertebrate and murine infection models. Our results suggest that flotillin assists in the assembly of protein complexes involved inS. aureusvirulence, and could thus be an attractive target for the development of new antimicrobial therapies.
中文翻译:

通过靶向 Flotillin 蛋白支架活性来减弱金黄色葡萄球菌的毒力
支架蛋白是普遍存在的分子伴侣,可结合蛋白质并促进多酶复合物的物理相互作用。在这里,我们使用生化方法来剖析多药耐药人类病原体金黄色葡萄球菌的 flotillin 同源蛋白 FloA 的支架活性。我们表明,FloA 促进膜蛋白复合物的寡聚化,例如膜相关的 RNase Rny,它构成称为降解体的 RNA 降解机制的一部分。缺乏 FloA 的细胞降低了 Rny 功能,并因此增加了负调节 S 的靶向 sRNA 转录物。金黄色葡萄球菌毒素的表达。改变 FloA 寡聚化的小分子也降低了 Rny 功能并降低了 S. 金黄色葡萄球菌在体外和体内,使用无脊椎动物和鼠感染模型。我们的结果表明,flotillin 有助于 S. 中涉及的蛋白质复合物的组装。金黄色葡萄球菌的毒力,因此可能成为开发新的抗菌疗法的一个有吸引力的目标。
更新日期:2017-07-22
中文翻译:

通过靶向 Flotillin 蛋白支架活性来减弱金黄色葡萄球菌的毒力
支架蛋白是普遍存在的分子伴侣,可结合蛋白质并促进多酶复合物的物理相互作用。在这里,我们使用生化方法来剖析多药耐药人类病原体金黄色葡萄球菌的 flotillin 同源蛋白 FloA 的支架活性。我们表明,FloA 促进膜蛋白复合物的寡聚化,例如膜相关的 RNase Rny,它构成称为降解体的 RNA 降解机制的一部分。缺乏 FloA 的细胞降低了 Rny 功能,并因此增加了负调节 S 的靶向 sRNA 转录物。金黄色葡萄球菌毒素的表达。改变 FloA 寡聚化的小分子也降低了 Rny 功能并降低了 S. 金黄色葡萄球菌在体外和体内,使用无脊椎动物和鼠感染模型。我们的结果表明,flotillin 有助于 S. 中涉及的蛋白质复合物的组装。金黄色葡萄球菌的毒力,因此可能成为开发新的抗菌疗法的一个有吸引力的目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号