当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined Theoretical and Experimental Studies of Nickel-Catalyzed Cross-Coupling of Methoxyarenes with Arylboronic Esters via C–O Bond Cleavage
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/jacs.7b04279 Martin C. Schwarzer 1, 2 , Ryosuke Konno 1, 2 , Takayuki Hojo 1, 2 , Akimichi Ohtsuki 1, 2 , Keisuke Nakamura 1, 2 , Ayaka Yasutome 1, 2 , Hiroaki Takahashi 1, 2 , Toshiaki Shimasaki 1, 2 , Mamoru Tobisu 1, 2 , Naoto Chatani 1, 2 , Seiji Mori 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/jacs.7b04279 Martin C. Schwarzer 1, 2 , Ryosuke Konno 1, 2 , Takayuki Hojo 1, 2 , Akimichi Ohtsuki 1, 2 , Keisuke Nakamura 1, 2 , Ayaka Yasutome 1, 2 , Hiroaki Takahashi 1, 2 , Toshiaki Shimasaki 1, 2 , Mamoru Tobisu 1, 2 , Naoto Chatani 1, 2 , Seiji Mori 1, 2
Affiliation
|
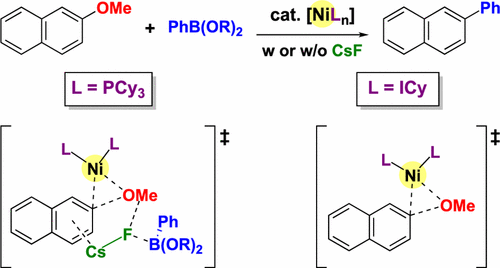
Nickel(0)-catalyzed cross-coupling of methoxyarenes through C–O bond activation has been the subject of considerable research because of their favorable features compared with those of the cross-coupling of aryl halides, such as atom economy and efficiency. In 2008, we have reported nickel/PCy3-catalyzed cross-coupling of methoxyarenes with arylboronic esters in which the addition of a stoichiometric base such as CsF is essential for the reaction to proceed. Recently, we have also found that the scope of the substrate in the Suzuki–Miyaura-type cross-coupling of methoxyarenes can be greatly expanded by using 1,3-dicyclohexylimidazol-2-ylidene (ICy) as the ligand. Interestingly, a stoichiometric amount of external base is not required for the nickel/ICy-catalyzed cross-coupling. For the mechanism and origin of the effect of the external base to be elucidated, density functional theory calculations are conducted. In the nickel/PCy3-catalyzed reactions, the activation energy for the oxidative addition of the C(aryl)–OMe bond is too high to occur under the catalytic conditions. However, the oxidative addition process becomes energetically feasible when CsF and an arylboronic ester interact with a Ni(PCy3)2/methoxyarene fragment to form a quaternary complex. In the nickel/ICy-catalyzed reactions, the oxidative addition of the C(aryl)–OMe bond can proceed more easily without the aid of CsF because the nickel-ligand bonds are stronger and therefore stabilize the transition state. The subsequent transmetalation from an Ar–Ni–OMe intermediate is determined to proceed through a pathway with lower energies than those required for β-hydrogen elimination. The overall driving force of the reaction is the reductive elimination to form the carbon–carbon bond.
中文翻译:

镍催化甲氧芳烃与芳硼酸酯通过C–O键断裂的交叉偶联的理论和实验研究
镍(0)催化的甲氧基芳烃通过C–O键活化的交叉偶联由于其与芳基卤化物交叉偶联的有利特性(如原子经济性和效率)而成为人们研究的热点。2008年,我们报告了镍/ PCy 3催化的甲氧基芳烃与芳基硼酸酯的交叉偶联,其中添加化学计量的碱(例如CsF)对于进行反应是必不可少的。最近,我们还发现,通过使用1,3-二环己基咪唑-2-亚基(ICy)作为配体,可以大大扩展Suzuki-Miyaura型甲氧基芳烃交叉偶联的底物范围。有趣的是,镍/ ICy催化的交叉偶联不需要化学计量的外部碱。为了阐明外部碱作用的机理和起源,进行了密度泛函理论计算。在镍/ PCy 3中-催化反应中,C(芳基)-OMe键氧化加成的活化能太高而无法在催化条件下发生。但是,当CsF和芳基硼酸酯与Ni(PCy 3)2相互作用时,氧化加成过程在能量上变得可行。/甲氧基芳烃片段形成季配合物。在镍/ ICy催化的反应中,不需要CsF就能更轻松地进行C(芳基)-OMe键的氧化加成,因为镍-配体键更强,因此稳定了过渡态。确定从Ar-Ni-OMe中间体进行的后续重金属化反应所经过的能量要比消除β-氢所需的能量低。反应的整体驱动力是还原消除,形成碳-碳键。
更新日期:2017-07-22
中文翻译:

镍催化甲氧芳烃与芳硼酸酯通过C–O键断裂的交叉偶联的理论和实验研究
镍(0)催化的甲氧基芳烃通过C–O键活化的交叉偶联由于其与芳基卤化物交叉偶联的有利特性(如原子经济性和效率)而成为人们研究的热点。2008年,我们报告了镍/ PCy 3催化的甲氧基芳烃与芳基硼酸酯的交叉偶联,其中添加化学计量的碱(例如CsF)对于进行反应是必不可少的。最近,我们还发现,通过使用1,3-二环己基咪唑-2-亚基(ICy)作为配体,可以大大扩展Suzuki-Miyaura型甲氧基芳烃交叉偶联的底物范围。有趣的是,镍/ ICy催化的交叉偶联不需要化学计量的外部碱。为了阐明外部碱作用的机理和起源,进行了密度泛函理论计算。在镍/ PCy 3中-催化反应中,C(芳基)-OMe键氧化加成的活化能太高而无法在催化条件下发生。但是,当CsF和芳基硼酸酯与Ni(PCy 3)2相互作用时,氧化加成过程在能量上变得可行。/甲氧基芳烃片段形成季配合物。在镍/ ICy催化的反应中,不需要CsF就能更轻松地进行C(芳基)-OMe键的氧化加成,因为镍-配体键更强,因此稳定了过渡态。确定从Ar-Ni-OMe中间体进行的后续重金属化反应所经过的能量要比消除β-氢所需的能量低。反应的整体驱动力是还原消除,形成碳-碳键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号