当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interparticle Reactions: An Emerging Direction in Nanomaterials Chemistry
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-07-20 00:00:00 , DOI: 10.1021/acs.accounts.7b00224 K. R. Krishnadas 1 , Ananya Baksi 1 , Atanu Ghosh 1 , Ganapati Natarajan 1 , Anirban Som 1 , Thalappil Pradeep 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-07-20 00:00:00 , DOI: 10.1021/acs.accounts.7b00224 K. R. Krishnadas 1 , Ananya Baksi 1 , Atanu Ghosh 1 , Ganapati Natarajan 1 , Anirban Som 1 , Thalappil Pradeep 1
Affiliation
|
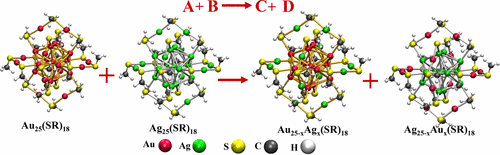
Nanoparticles exhibit a rich variety in terms of structure, composition, and properties. However, reactions between them remain largely unexplored. In this Account, we discuss an emerging aspect of nanomaterials chemistry, namely, interparticle reactions in solution phase, similar to reactions between molecules, involving atomically precise noble metal clusters. A brief historical account of the developments, starting from the bare, gas phase clusters, which led to the synthesis of atomically precise monolayer protected clusters in solution, is presented first. Then a reaction between two thiolate-protected, atomically precise noble metal clusters, [Au25(PET)18]− and [Ag44(FTP)30]4– (PET = 2-phenylethanethiol, FTP = 4-fluorothiophenol), is presented wherein these clusters spontaneously exchange metal atoms, ligands, and metal–ligand fragments between them under ambient conditions. The number of exchanged species could be controlled by varying the initial compositions of the reactant clusters. Next, a reaction of [Au25(PET)18]− with its structural analogue [Ag25(DMBT)18]− (DMBT = 2,4-dimethylbenzenethiol) is presented, which shows that atom-exchange reactions happen with structures conserved. We detected a transient dianionic adduct, [Ag25Au25(DMBT)18(PET)18]2–, formed between the two clusters indicating that this adduct could be a possible intermediate of the reaction. A reaction involving a dithiolate-protected cluster, [Ag29(BDT)12]3– (BDT = 1,3-benzenedithiol), is also presented wherein metal atom exchange alone occurs, but with no ligand and fragment exchanges. These examples demonstrate that the nature of the metal–thiolate interface, that is, its bonding network and dynamics, play crucial roles in dictating the type of exchange processes and overall rates. We also discuss a recently proposed structural model of these clusters, namely, the Borromean ring model, to understand the dynamics of the metal–ligand interfaces and to address the site specificity and selectivity in these reactions.
中文翻译:

粒子间反应:纳米材料化学的新兴方向
纳米颗粒在结构,组成和性能方面表现出丰富的多样性。但是,它们之间的反应在很大程度上尚待探索。在该帐户中,我们讨论纳米材料化学的一个新兴的方面,即,在溶液相反应间,类似于分子之间的反应,涉及原子精确贵金属簇。首先简要介绍了从裸露的气相簇开始的发展情况,这导致了溶液中原子精确的单层保护簇的合成。然后,两个硫醇盐保护的原子精确的贵金属簇[Au 25(PET)18 ] -和[Ag 44(FTP)30 ]之间发生反应提出了4-(PET = 2-苯基乙硫醇,FTP = 4-氟硫酚),其中这些簇在环境条件下自发交换金属原子,配体和金属-配体碎片。可以通过改变反应物簇的初始组成来控制交换物质的数量。接下来,提出了[Au 25(PET)18 ] -与结构类似物[Ag 25(DMBT)18 ] -(DMBT = 2,4-二甲基苯硫醇)的反应,这表明原子交换反应在结构保守的情况下发生。我们检测到一种短暂的双阴离子加合物[Ag 25 Au 25(DMBT)18(PET)在两个簇之间形成的18 [ 18 ] 2–表示该加合物可能是反应的可能中间体。涉及二硫醇盐保护簇[Ag 29(BDT)12 ] 3–的反应也存在(BDT = 1,3-苯二硫醇),其中仅发生金属原子交换,而没有配体和片段交换。这些例子表明,金属-硫醇盐界面的性质,即其键合网络和动力学,在决定交换过程的类型和总速率方面起着至关重要的作用。我们还讨论了最近提出的这些簇的结构模型,即Borromean环模型,以了解金属-配体界面的动力学并解决这些反应中的位点特异性和选择性。
更新日期:2017-07-20
中文翻译:

粒子间反应:纳米材料化学的新兴方向
纳米颗粒在结构,组成和性能方面表现出丰富的多样性。但是,它们之间的反应在很大程度上尚待探索。在该帐户中,我们讨论纳米材料化学的一个新兴的方面,即,在溶液相反应间,类似于分子之间的反应,涉及原子精确贵金属簇。首先简要介绍了从裸露的气相簇开始的发展情况,这导致了溶液中原子精确的单层保护簇的合成。然后,两个硫醇盐保护的原子精确的贵金属簇[Au 25(PET)18 ] -和[Ag 44(FTP)30 ]之间发生反应提出了4-(PET = 2-苯基乙硫醇,FTP = 4-氟硫酚),其中这些簇在环境条件下自发交换金属原子,配体和金属-配体碎片。可以通过改变反应物簇的初始组成来控制交换物质的数量。接下来,提出了[Au 25(PET)18 ] -与结构类似物[Ag 25(DMBT)18 ] -(DMBT = 2,4-二甲基苯硫醇)的反应,这表明原子交换反应在结构保守的情况下发生。我们检测到一种短暂的双阴离子加合物[Ag 25 Au 25(DMBT)18(PET)在两个簇之间形成的18 [ 18 ] 2–表示该加合物可能是反应的可能中间体。涉及二硫醇盐保护簇[Ag 29(BDT)12 ] 3–的反应也存在(BDT = 1,3-苯二硫醇),其中仅发生金属原子交换,而没有配体和片段交换。这些例子表明,金属-硫醇盐界面的性质,即其键合网络和动力学,在决定交换过程的类型和总速率方面起着至关重要的作用。我们还讨论了最近提出的这些簇的结构模型,即Borromean环模型,以了解金属-配体界面的动力学并解决这些反应中的位点特异性和选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号