当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Covalent Enzyme Inhibition through Fluorosulfate Modification of a Noncatalytic Serine Residue
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-07-20 00:00:00 , DOI: 10.1021/acschembio.7b00403 Olugbeminiyi O. Fadeyi 1 , Lise R. Hoth 1 , Chulho Choi 1 , Xidong Feng 1 , Ariamala Gopalsamy 2 , Erik C. Hett 2 , Robert E. Kyne 1 , Ralph P. Robinson 1 , Lyn H. Jones 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-07-20 00:00:00 , DOI: 10.1021/acschembio.7b00403 Olugbeminiyi O. Fadeyi 1 , Lise R. Hoth 1 , Chulho Choi 1 , Xidong Feng 1 , Ariamala Gopalsamy 2 , Erik C. Hett 2 , Robert E. Kyne 1 , Ralph P. Robinson 1 , Lyn H. Jones 2
Affiliation
|
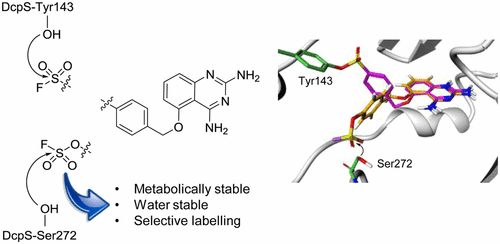
Irreversible enzyme inhibitors and covalent chemical biology probes often utilize the reaction of a protein cysteine residue with an appropriately positioned electrophile (e.g., acrylamide) on the ligand template. However, cysteine residues are not always available for site-specific protein labeling, and therefore new approaches are needed to expand the toolkit of appropriate electrophiles (“warheads”) that target alternative amino acids. We previously described the rational targeting of tyrosine residues in the active site of a protein (the mRNA decapping scavenger enzyme, DcpS) using inhibitors armed with a sulfonyl fluoride electrophile. These inhibitors subsequently enabled the development of clickable probe technology to measure drug-target occupancy in live cells. Here we describe a fluorosulfate-containing inhibitor (aryl fluorosulfate probe (FS-p1)) with excellent chemical and metabolic stability that reacts selectively with a noncatalytic serine residue in the same active site of DcpS as confirmed by peptide mapping experiments. Our results suggest that noncatalytic serine targeting using fluorosulfate electrophilic warheads could be a suitable strategy for the development of covalent inhibitor drugs and chemical probes.
中文翻译:

通过非硫酸化丝氨酸残基的氟硫酸盐修饰的共价酶抑制。
不可逆酶抑制剂和共价化学生物学探针通常利用蛋白质半胱氨酸残基与适当定位的亲电子试剂(例如,(丙烯酰胺)。然而,半胱氨酸残基并不总是可用于位点特异性蛋白质标记,因此需要新的方法来扩展针对替代氨基酸的合适亲电试剂(“战斗部”)的工具包。我们先前描述了使用带有磺酰氟亲电试剂的抑制剂对蛋白质(mRNA脱盖清除剂,DcpS)的活性位点中酪氨酸残基的合理靶向。这些抑制剂随后促成了可点击探针技术的发展,以测量活细胞中药物靶标的占有率。在这里,我们描述了一种含氟硫酸盐的抑制剂(芳基氟硫酸盐探针(FS-p1)),具有出色的化学和代谢稳定性,可与DcpS相同活性位点中的非催化丝氨酸残基选择性反应,如肽图分析实验所证实。我们的结果表明,使用氟硫酸亲电子战斗部进行非催化丝氨酸靶向可能是开发共价抑制剂药物和化学探针的合适策略。
更新日期:2017-07-21
中文翻译:

通过非硫酸化丝氨酸残基的氟硫酸盐修饰的共价酶抑制。
不可逆酶抑制剂和共价化学生物学探针通常利用蛋白质半胱氨酸残基与适当定位的亲电子试剂(例如,(丙烯酰胺)。然而,半胱氨酸残基并不总是可用于位点特异性蛋白质标记,因此需要新的方法来扩展针对替代氨基酸的合适亲电试剂(“战斗部”)的工具包。我们先前描述了使用带有磺酰氟亲电试剂的抑制剂对蛋白质(mRNA脱盖清除剂,DcpS)的活性位点中酪氨酸残基的合理靶向。这些抑制剂随后促成了可点击探针技术的发展,以测量活细胞中药物靶标的占有率。在这里,我们描述了一种含氟硫酸盐的抑制剂(芳基氟硫酸盐探针(FS-p1)),具有出色的化学和代谢稳定性,可与DcpS相同活性位点中的非催化丝氨酸残基选择性反应,如肽图分析实验所证实。我们的结果表明,使用氟硫酸亲电子战斗部进行非催化丝氨酸靶向可能是开发共价抑制剂药物和化学探针的合适策略。










































 京公网安备 11010802027423号
京公网安备 11010802027423号