当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of Donor–Acceptor Cyclopropanes with Naphthoquinones: Redox and Lewis Acid Catalysis Working in Concert
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-19 07:51:06 , DOI: 10.1002/anie.201703732 Alexander Lücht 1 , Lukas J. Patalag 1 , André U. Augustin 1 , Peter G. Jones 2 , Daniel B. Werz 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-19 07:51:06 , DOI: 10.1002/anie.201703732 Alexander Lücht 1 , Lukas J. Patalag 1 , André U. Augustin 1 , Peter G. Jones 2 , Daniel B. Werz 1
Affiliation
|
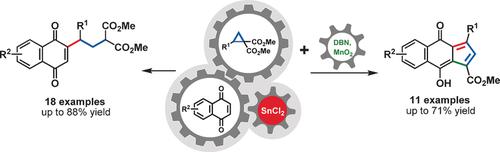
Reactions of 2-arylcyclopropane dicarboxylates with naphthoquinones are reported. The key feature was the use of catalytic amounts of SnCl2, which acts as both an electron donor and a Lewis acid. By an in situ umpolung of naphthoquinone the formerly electrophilic species is converted into a nucleophile that is able to trigger the ring-opening of the three-membered ring with formation of a new C−C bond. Treatment of these products with base under oxidative conditions resulted—through loss of methyl formate—in cyclopentannulated products with fully conjugated π systems exhibiting intensive absorptions in the visible range.
中文翻译:

供体-受体环丙烷与萘醌的反应:氧化还原和路易斯酸催化协同作用
报道了2-芳基环丙烷二羧酸酯与萘醌的反应。关键特征是使用催化量的SnCl 2,它既充当电子给体,又充当路易斯酸。通过原位萘醌的聚醚酮,以前的亲电物质被转化为亲核试剂,该亲核试剂能够触发三元环的开环并形成新的C-C键。这些产物在氧化条件下用碱处理,是由于甲酸甲酯的损失,导致了在完全可见光范围内具有强吸收作用的完全共轭π系统的环戊烯化产品。
更新日期:2017-07-20
中文翻译:

供体-受体环丙烷与萘醌的反应:氧化还原和路易斯酸催化协同作用
报道了2-芳基环丙烷二羧酸酯与萘醌的反应。关键特征是使用催化量的SnCl 2,它既充当电子给体,又充当路易斯酸。通过原位萘醌的聚醚酮,以前的亲电物质被转化为亲核试剂,该亲核试剂能够触发三元环的开环并形成新的C-C键。这些产物在氧化条件下用碱处理,是由于甲酸甲酯的损失,导致了在完全可见光范围内具有强吸收作用的完全共轭π系统的环戊烯化产品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号